| Product Description | This kit is suitable for recovering up to 8 μ GDNA (70bp-10kb), the recovery was 60-85%. The agarose gel melts in a mild buffer (De-a solution), in which the protective agent can prevent the degradation of linear DNA at high temperature, and then selectively bind DNA to the membrane under the action of de-b solution. The purified DNA has high purity and maintains fragment integrity and high biological activity, and can be directly used in molecular biology experiments such as ligation, in vitro transcription, PCR amplification, sequencing, microinjection, etc Component: 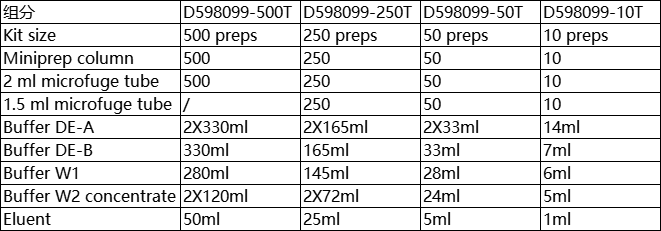
Component remarks: Buffer DE-A : gel melting agent, containing DNA protective agent, preventing DNA degradation at high temperature, sealed storage at room temperature ;
Buffer DE-B : binding solution ( to promote the selective binding of DNA fragments greater than 70bp to DNA preparation membrane ), sealed storage at room temperature ;
Buffer W1 : washing liquid, sealed storage at room temperature ;
Buffer W2 concentrate : desalted liquid, before use, add anhydrous ethanol ( 100 % ethanol or 95 % ethanol ) according to the volume specified on the reagent bottle, mix evenly, and store in airtight at room temperature ;
Eluent : eluent, sealed storage at room temperature. Points for attention: 1.Buffer DE-A ( containing-alkyl alcohol ) Buffer DE-B and Buffer W1 contain irritating compounds. Wear latex gloves and glasses during operation to avoid contamination of skin, eyes and clothes, and beware of inhalation of mouth and nose. If the skin, eyes, to immediately rinse with a lot of water or saline, if necessary, seek medical advice ; 2.In step 1, cutting the gel into small pieces can greatly shorten the melting time of the gel ( linear DNA is easily hydrolyzed under high temperature conditions for a long time ), thereby increasing the recovery rate. Do not expose the gel containing DNA to ultraviolet light for a long time to reduce the damage caused by ultraviolet light to DNA. 3.In step 2, the gel must be completely melted, otherwise it will seriously affect the DNA recovery rate ; 4.Heating Eluent or deionized water to 65 ° C is beneficial to improve the elution efficiency ; 5.DNA molecules are acidic, and it is recommended to preserve in 2.5mM Tris-HCl, pH 7.0-8.5 eluent. Experimental preparation:
Before the first use, add a specified volume of anhydrous ethanol to Buffer W2 concentrate.
2. Prepare tip heads and centrifuge tubes free from nucleic acid and nucleic acid contamination
3. Prepare a 75 ° C water bath.
4. Before use, check if there is any precipitation in Buffer DE-B. If precipitation occurs, it should be heated and melted in a 70 ° C temperature bath and cooled to room temperature before use. Operation steps:
1. Cut the agarose gel containing the target DNA under the UV lamp, suck up the liquid on the surface of the gel with a paper towel and cut it into pieces. Calculate the weight of gel (record the weight of 1.5 ml centrifuge tube in advance), which is taken as a volume of gel (e.g. 100 mg=100 μ L volume).
2. Add three gel volumes of Buffer DE-A, heat them at 75C after mixing (low melting point agarose gel is heated at 40), intermittently mix them (every 2-3 min) until the gel block is completely melted (about 6-8 min), and Buffer DE-A is a red solution. In the process of melting gel, it can help to observe whether the gel is completely melted.
3. Add 0.5 buffer DE-A volumes of buffer DE-B and mix evenly. When the isolated DNA fragment is less than 400 bp, one more gel volume of isopropanol shall be added.
*After adding Buffer DE-B, the color of the mixture turns yellow. Mix thoroughly to ensure the formation of a uniform yellow solution.
Steps 4-6 can choose between negative pressure method or centrifugal method.
A. Negative pressure method
4A. Connect the negative pressure device correctly and insert the DNA preparation tube into the socket of the negative pressure device. Suck the mixture from step 3, transfer it to the preparation tube, open and adjust the negative pressure to -25-30 inches of mercury, and slowly remove the solution from the tube.
5A. Add 500 l of Buffer W1 and absorb the intermediate solution.
6A. Add 700 ul Buffer W2 and absorb the intermediate solution. Wash again with 700 l of Buffer W2 using the same method, and confirm that anhydrous ethanol has been added to the buffer W2 concentrate according to the specified volume on the reagent bottle.
·Two washes with Buffer W2 can ensure complete removal of salt and eliminate any impact on subsequent experiments.
7A. Place the preparation tube in a 2ml centrifuge tube (provided by the reagent kit) and centrifuge 12000xg for 1 minute. B. Centrifuge method
4B. Take the mixture from step 3 and transfer it to a DNA preparation tube (placed in an m centrifuge tube (provided in the reagent). Centrifuge at 12000Xg for 1 minute. Discard the filtrate.
5B. Place the preparation tube back into a 2 ml centrifuge tube, add 500 l Buffer W1, centrifuge 12000 Xg for 30 seconds, and discard the filtrate.
6B. Place the preparation tube back into a 2 ml centrifuge tube, add 700 l Buffer W2, centrifuge 12000 Xg for 30 seconds, and discard the filtrate. Using the same method, wash once with 700 ul Buffer W2 and centrifuge 12000xg for 1 minute., Confirm that anhydrous ethyl alcohol has been added to the designated volume on the vials in Buffer W2 concentrate twice. Rinsing with Buffer W2 can ensure complete removal of salt and eliminate any impact on subsequent experiments.
7B, place the preparation tube back into a 2ml centrifuge tube and centrifuge 12000 Xg for 1 minute.
8. Place the preparation tube in a clean 1.5m centrifuge tube, add 25-30 Euent or deionized water in the center of the preparation membrane, and let it stand at room temperature for 1 minute. Wash DNA by centrifugation at 12000xg for 1 minute.
Heating Elent or deionized water to 65C will improve the elution efficiency. 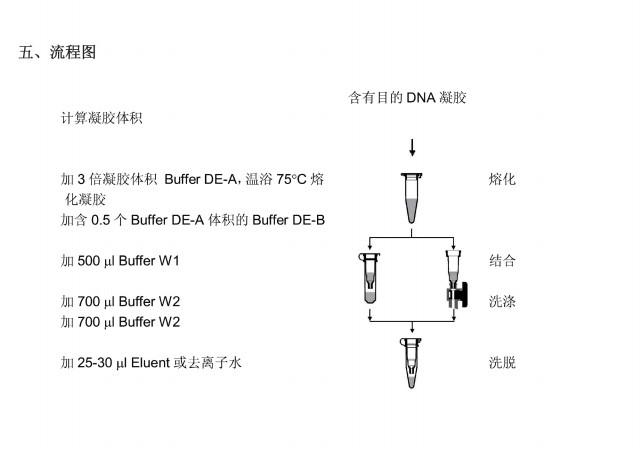
Scope of application: Nucleic acid extraction and purification |
|---|



