1,3-Thiazole building blocks in natural products and synthetic materials

Thiazole, also known as 1,3-thiazole, is a five-membered heterocyclic compound containing both sulfur and nitrogen. The term "thiazole" also refers to a large group of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS.[1,2] The thiazole ring is one of the key components of the vitamin thiamine (B1).
Molecular and electronic structure
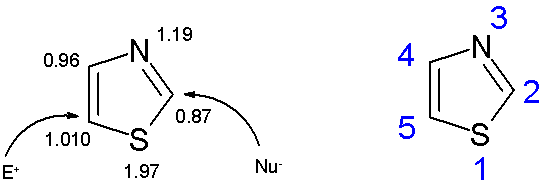
Thiazole is a member of the azole group of compounds, in addition to imidazole and oxazole. The thiazole ring is planar and aromatic. Thiazoles are characterized by a larger π-electron departure domain than the corresponding oxazole and therefore have greater aromaticity. This aromaticity is also evidenced by the chemical shifts of hydrogen (between 7.27 and 8.77 ppm) in the HNMR spectra, significantly indicating a strong antimagnetic ring current. The calculated π-electron densities label C5 as the main site of electrophilic substitution and C2 as the site of nucleophilic substitution.
Occurrence of thiazoles and thiazolium salts
The thiazole functional group is an important building block of vitamin B1 and ebomycin. Other more important thiazole compounds include its thickened ring derivatives with benzene, known as benzothiazoles, which are present in the chemical fluorescein of fireflies.
When the nitrogen atom in the thiazole is alkylated, a thiazole salt is formed. The thiazole salt serves as a catalyst in the Stettel reaction and in the condensation of benzoin. Thiazole dyes are also used to dye cotton.

Thiazoles are highly abundant in biological macromolecules, whereas oxazoles, in which the oxygen atom replaces the sulfur atom, are not. It is present in naturally occurring peptides and has been used to develop peptidomimetic molecules (i.e., molecules that mimic the function and structure of peptides) [3].
Application
Substituted 1,3-thiazole structures are ubiquitous in natural product and synthetic material applications.[4]

Fig 1. The occurrence of the thiazole ring in natural products and synthetic molecules
Thiazole-containing natural products isolated from cyanobacteria of different marine organisms include small molecule linear peptides (e.g., Nordysidenin 1 in Fig. 1), linear oligopeptides (known as Apramidas), cyclic peptides (e.g., Tenuecyclamide A 2 shown in Fig. 1), and alkaloids. [5] In addition, a number of macrocyclic compounds and cyclic lactones containing thiazole rings have been found in some soil bacterial extracts. [6]
1,3-Thiazole is a particular building block in the drug discovery phase. Many thiazole-based compounds have been shown to have antitumor, antifungal, and enzyme inhibitory activities;[7] some of which are currently approved drugs. [8] 1,3-Thiazoles are also indispensable in organic synthesis, serving as immobilizing groups for formyl functional groups and as chiral auxiliaries in asymmetric reactions. [9,10]
1,3-Thiazole derivatives as fluorescent dyes (e.g., Thiazole Orange 3) have found important applications in cytology as "light-up" probes of DNA. This technology benefits from thiazole-containing semiconducting conjugated polymers[11] as well as catalysts such as compound 4[12] in Fig. 1.
Reference
1. Zoltewicz, J. A.; Deady, L. W. (1978). Quaternization of Heteroaromatic Compounds. Quantitative Aspects. Advances in Heterocyclic Chemistry. Vol. 22. pp. 71-121. doi:10.1016/S0065-2725(08)60103-8. ISBN 9780120206223.
2. Eicher, T.; Hauptmann, S. (2003). The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications. ISBN 978-3-527-30720-3.
3. Mak, Jeffrey Y. W.; Xu, Weijun; Fairlie, David P. (2015-01-01). Peptidomimetics I (PDF). Topics in Heterocyclic Chemistry. Vol. 48. Springer Berlin Heidelberg. pp. 235–266. doi:10.1007/7081_2015_176. ISBN 978-3-319-49117-2.
4. Chen B.; Heal, W. In Comprehensive Heterocyclic Chemistry, 3rd edition, eds. A.R. Katritzky, C.A. Ramsden, E.F.V. Scriven, and R. J.K. Taylor Pergamon, Oxford, 2008. Vol. 4, p. 635.
5. König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. ChemBioChem 2006, 7, 229.
6. Hofle, G.H.; Bedorf, N.; Steinmetz, H.; Schomburg, D.; Gerth, K.; Reichenbach, H. Angew. Chem. Int. Ed. 1996, 35, 1567.
7. (a) Kumar, S.; Patil, M. T.; Kataria, R.; Salunke, D. B. Eds. Gupta, G. K.; Vinod, K. Chem. Drug Design 2016, 243-281. (b) Thorat, B. R.; Joshi, V.; Thorat, V. B. Heterocycl. Lett. 2016, 6, 389-409.
8. According to www.drugbank.ca as of September 2019 there are 28 thiazole-containing marketed drugs. These drugs include natural products, natural-product-based architectures, and purely synthetic compounds.
9. Dondoni, A.; Marra, A. Chem. Rev. 2004, 104, 2557.
10. Velazquez, F.; Olivo, H.F. Curr. Org. Chem. 2002, 6, 303.
11. (a) Subramaniyan, S.; Kim, F.S.; Ren, G.; Li, H.; Jenekhe, S.A. Macromolecules 2012, 45, 9029. (b) Hussain, S.; De, S.; Iyer, P.K. ACS Appl. Mater. Interfaces 2013, 5, 2234.
(a) Luo, Q.-L.; Tan, J.-P.; Li, Z.-F.; Nan, W.-H.; Xiao, D.-R. J. Org. Chem. 2012, 77, 8332. (b) Frija, L. M. T.; Pombeiro, A. J. L.; Kopylovich, M. N. Coord. Chem. Rev. 2016, 308, 32-55.
Aladdin:https://www.aladdinsci.com
List of related products
