1,4-Cyclohexadiene, CHD

Sandra Forbes
Product Manager
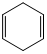
1,4-Cyclohexadiene can act as hydrogen atom donor due to aromatization as driving force.
Recent Literature

(Bis(dimethylamino)carbazole) was used as photocatalyst in the reduction of unactivated aryl chlorides and alkyl chlorides in the presence of CHD as hydrogen atom donor at room temperature. The catalytic system can also be applied to the coupling of aryl chlorides with electron-rich arene and heteroarenes to affect C-C bond-forming reactions.
R. Matsubara, T. Yabuta, U. M. Idros, M. Hayashi, F. Ema, Y. Kobori, K. Sakata, J. Org. Chem., 2018, 83, 9381-9390.
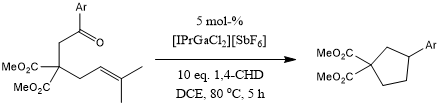
[IPr·GaCl2][SbF6] catalyzes a tandem process involving a ring-closing carbonyl-olefin metathesis and a transfer hydrogenation in the presence of 1,4-cyclohexadiene as an H2 surrogate to reduce the cyclic alkene intermediates. This stereoselective reaction leads to 1,2-cis-disubstituted cyclopentanes and various cyclohexanes.
A. Djurovic, M. Vayer, Z. Li, R. Guillot, J.-P. Baltaze, V. Gandon, C. Bour, Org. Lett., 2019, 21, 8132-8137.
DOI: 10.1021/acs.orglett.9b03240
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/1,4-cyclohexadiene-chd.shtm
Aladdinsci: https://www.aladdinsci.com/
