108 SadPhos, Find Your Own Chiral Phosphine Ligands/Catalysts
Sandra Forbes
Product Manager

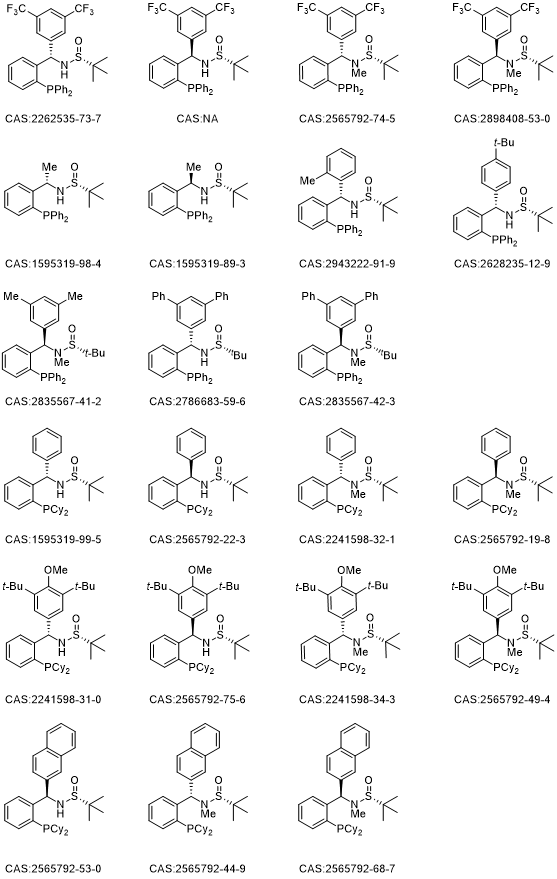
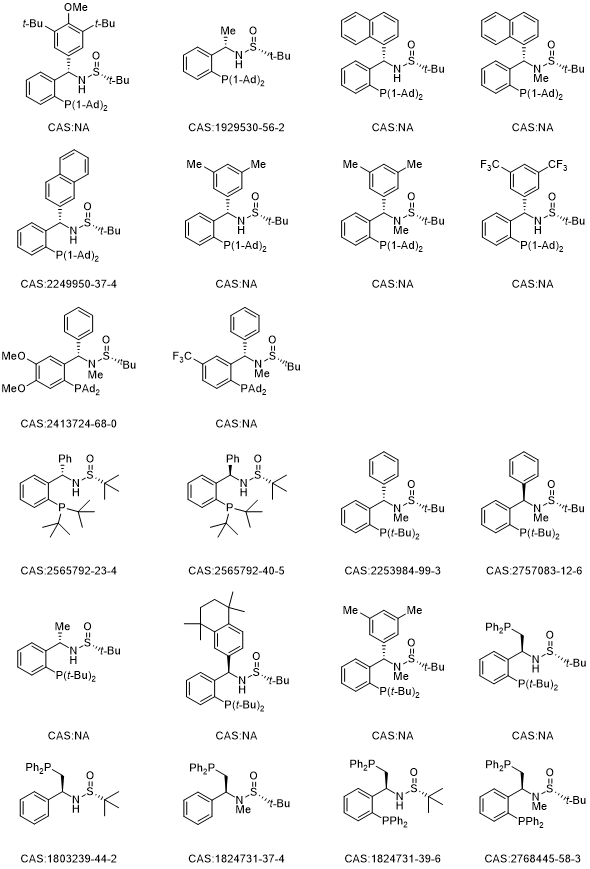
12 series of chiral tert-butylsulfinamide phosphine ligands/catalysts (Sulfinamide Phosphine, collectively referred to as SadPhos) based on the design concept of non-C2 symmetry have been developed , combining rigidity and flexibility, as well as incorporating both soft and hard coordinating atoms (O, S, N, P). These SadPhos ligands possess numerous advantages such as structural diversity, ease of modification, and scalability for kilogram-scale preparation. SadPhos has demonstrated excellent catalytic performance and enantioselectivity in asymmetric catalysis with transition metals and organocatalysis with small organic molecules. This introduction of the "108 Heroes" covers a range of phosphine ligands including Ming-Phos, Xu-Phos, PC-Phos, Xiao-Phos, Wei-Phos, Xiang-Phos, TY-Phos, Peng-Phos, and more.

In transition metal-catalyzed reactions, Ming-Phos, Xu-Phos, PC-Phos, Xiao-Phos, Wei-Phos, Xiang-Phos, TY-Phos, and others, as mono-phosphine ligands, P, N-ligands, P, S-ligands, and P, O-ligands, can adopt adaptive coordination modes with eight transition metals including gold, copper, palladium, iridium, nickel, rhodium, silver, and cobalt, demonstrating remarkable catalytic efficiency and asymmetric induction ability. Currently, they have been efficiently applied in Suzuki coupling, olefin diamination, arylation of phosphites, hydroarylation reactions, asymmetric hydrogenation, Heck/etherification, olefin carboboration, carbonylation, olefin arylation/alkynylation, C-S cross-coupling, C-H arylation, allene γ-addition, olefin reductive Heck, olefin carbon iodination, photocatalytic coupling, carbene arylsilylation, allene C-P coupling, alkyne hydrophosphination, olefin diarylation, olefin carbon oxidation, allene boration/carbonylation, Heck/Tsuji-Trost reactions, cycloaddition reactions, polymerization reactions, and more.
In organic small-molecule catalyzed reactions, Xiao-Phos, Wei-Phos, Peng-Phos, and others, which provide hydrogen donors through sulfinamides, are highly efficient multifunctional chiral tertiary phosphine catalysts. They have been successfully applied in asymmetric intramolecular and intermolecular Rauhut-Currier reactions, γ-addition reactions of electron-deficient imines with allenoates, asymmetric [4+2] cycloaddition reactions involving ketenes or allenoates, difunctionalization reactions of α,β-unsaturated ketones with TMSN3, dearomatization [3+2] cycloaddition reactions of 3-nitroindoles with allenoates, and asymmetric allylation reactions, achieving excellent reaction results.
![]()

References
1. Shutao Qi, Wenshao Ye, Yunkai Hua, Liangkai Pan, Junfeng Yang*, and Junliang Zhang*, Ligand-enabled palladium-catalysed enantioselective synthesis of α-quaternary amino and glycolic acids derivatives. Nat. Synth. 2023, DOI: 10.1038/s44160-023-00448-7.
2. Yue Sun, Kaida Zhou, Chun Ma, Zhiming Li*, and Junliang Zhang*, Rhodium/Ming-Phos-catalyzed asymmetric annulation reaction of silacyclobutanes with terminal alkynes. Green Synth. Catal. 2023, DOI: 10.1016/j.gresc.2023.09.001.
3. Hui Zhang, Bing Xu, Liejin Zhou , Zhan-Ming Zhang*, and Junliang Zhang*, Polymer-supported chiral palladium-based complexes as efficient heterogeneous catalysts for asymmetric reductive Heck reaction. Green Synth. Catal. 2023, DOI: 10.1016/j.gresc.2023.04.002.
4. Li-Ming Zhang, Wenjun Luo, Jiangzhen Fu, Yu Liu*, and Junliang Zhang*, W-Phos Ligand Enables Copper-Catalyzed Enantioselective Alkylation of N-Sulfonyl Ketimines with Grignard Reagents, ACS Catal. 2023, 13, 13, 8830–8837. DOI: 10.1021/acscatal.3c01775.
5. Jie Han†, Siyuan Liu†, Huanan Wang, Jie Wang, Hui Qian, Zhiming Li*, Shengming Ma*,Junliang Zhang*, Pd/Xu-Phos-catalyzed asymmetric elimination of fully substituted enol triflates into axially chiral trisubstituted allenes, Sci. Adv. 2023, 9, eadg1002. DOI: 10.1126/sciadv.adg1002.
6. Shuting Zhang, Shuaijie Wu, Qiang Wang, Shiji Xu, Ying Han, Chao-Guo Yan, Junliang Zhang*, Lei Wang*, Enantioselective Synthesis of Dihydropyrazoles by Palladium/Xu-Phos-Catalyzed Alleneamination of β, γ-Unsaturated Hydrazones with Propargylic Acetates, Angew. Chem.Int. Ed. 2023, 62, e202300309, DOI: 10.1002/anie.202300309.
7. Youshao Tu, Bing Xu, Qian Wang, Honglin Dong, Zhan-Ming Zhang*, Junliang Zhang*, Palladium/TY-Phos-Catalyzed Asymmetric Heck/Tsuji–Trost Reaction of o-Bromophenols with 1,3-Dienes, J. Am. Chem. Soc. 2023, DOI: 10.1021/jacs.2c12752.
8. Chun Ma, Yue Sun, Junfeng Yang*, Hao Guo*, and Junliang Zhang*, Catalytic Asymmetric Synthesis of Tröger’s Base Analogues with Nitrogen Stereocenter, ACS Cent. Sci. 2023, DOI: 10.1021/acscentsci.2c01121.
9. Yin Yuan, Junfeng Yang* and Junliang Zhang*, Cu-catalyzed enantioselective decarboxylative cyanation via the synergistic merger of photocatalysis and electrochemistry. Chem. Sci., 2023, DOI: 10.1039/D2SC05428K.
10. Shiquan Gao, Chen Wang, Junfeng Yang* & Junliang Zhang*, Cobalt-catalyzed enantioselective intramolecular reductive cyclization via electrochemistry. Nat. Commun. 2023, 14, 1301. DOI: 10.1038/s41467-023-36704-9.
11. Ronghua Zhang, Shan Xu, Zhou Luo, Yuanyuan Liu,* and Junliang Zhang*, Enantiodivergent Hydrogenation of Exocyclic α, β-Unsaturated Lactams Enabled by Switching the N-Chirality of Iridium Catalyst. Angew. Chem. Int. Ed. 2023, e202213600, DOI: 10.1002/anie.202213600.
Aladdinsci: https://www.aladdinsci.com/