2,3-Dichloro-5,6-Dicyanobenzoquinone, DDQ

Product Manager:Nick Wilde
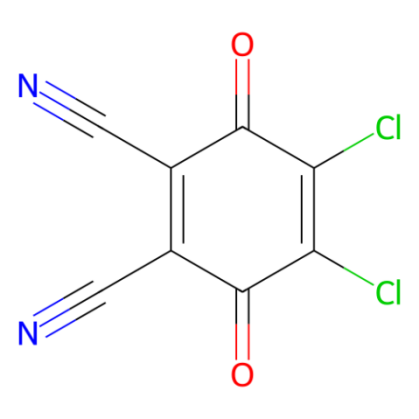
DDQ (2,3-dichloro-5,6-dicyanobenzoquinone), which exhibits stronger oxidizing properties than 1,4-benzoquinone, serves as a reagent in oxidative coupling and cyclization reactions, as well as in the dehydrogenation of hydroaromatic compounds.
Recent Literature
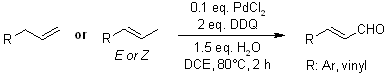
Palladium-catalyzed oxygenation of allyl arenes or alkenes efficiently produces (E)-alkenyl aldehydes, with allylic C-H bond cleavages occurring under mild conditions. Mechanistic investigations reveal that water serves as the oxygen source in this process.
H. Chen, H. Jiang, C. Cai, J. Dong, W. Fu, Org. Lett., 2011, 13, 992-994.
https://doi.org/10.1021/ol1030316
![]()
Alkyl nitrites were synthesized in high to excellent yields through the reaction of alcohols and thiols with a combination of triphenylphosphine, 2,3-dichloro-5,6-dicyanobenzoquinone, and Bu4NNO2 in acetonitrile. This approach enables the selective transformation of primary alcohols, even in the presence of secondary and tertiary alcohols and thiols.
B. Akhlaghinia, A. R. Pourali, Synthesis, 2004, 1747-1749.
https://doi.org/10.1055/s-2004-829122
The deprotection of benzyl ethers was efficiently achieved in the presence of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in acetonitrile under irradiation with long wavelength UV light.
M. A. Rahim, S. Matsumura, K. Toshima, Tetrahedron Lett., 2005, 46, 7307-7309.
https://doi.org/10.1016/j.tetlet.2005.08.132
A mild and highly functional group-tolerant method for photoinduced oxidative N-dealkylation of both aryl tertiary amines and amides was achieved in an alkaline environment.
Q. Lian, J. Chen, K. Huang, K. Hou, J. Fang, W. Wei, J. Zhou, Org. Lett., 2023, 25, 8387-8392.
https://doi.org/10.1021/acs.orglett.3c03519
A tandem process involving TBAB-catalyzed substitution followed by a novel oxidative rearrangement enables the synthesis of aryl or alkenyl nitriles from benzyl and allyl halides. The broad applicability and mild conditions of these methods suggest their potential utility in organic synthesis.
W. Zhou, J. Xu, L. Zhang, N. Jiao, Org. Lett., 2010, 12, 2888-2891.
https://doi.org/10.1021/ol101094u
An affordable homogeneous iron catalyst facilitates a direct synthesis of alkenyl nitriles from allylarenes or alkenes. This process involves three C-H bond cleavages under mild conditions, with the cleavage of the allyl C(sp3)-H bond serving as the rate-limiting step.
C. Qin, N. Jiao, J. Am. Chem. Soc., 2010, 132, 15893-15895.
https://doi.org/10.1021/ja1070202
An iron-catalyzed oxidative dehydrogenation allows for the α-arylation of deoxybenzoins with a wide range of non-prefunctionalized arenes, exhibiting excellent functional group tolerance. This reaction offers an efficient route to synthetically valuable 1,2,2-triarylethanones.
T. Chen, Y.-F. Li, Y. An, F.-M. Zhang, Org. Lett., 2016, 18, 4754-4757.
https://doi.org/10.1021/acs.orglett.6b02516
Iron catalyzes an oxidative coupling reaction for the α-amination of ketones with free sulfonamides, eliminating the need for prefunctionalization of either reactant. Both primary and secondary sulfonamides serve as effective coupling partners in this process.
F. Song, S. H. Park, C. Wu, A. E. Strom, J. Org. Chem., 2023, 88, 3353-3358.
https://doi.org/10.1021/acs.joc.3c00210
An efficient one-pot synthesis of pyrrole-2-carboxylates and -carboxamides from chalcones and glycine esters or amides involves an electrocyclic ring closure as the key step. The resulting 3,4-dihydro-2H-pyrrole intermediates are then oxidized to the corresponding pyrroles using stoichiometric oxidants or catalytic copper(II) and air, yielding good product quantities.
D. Imbri, N. Netz, M. Kucukdisli, L. M. Kammer, P. Jung, A. Kretzschmann, T. Opatz, J. Org. Chem., 2014, 79, 11750-11758.
https://doi.org/10.1021/jo5021823
A reliable approach for the direct synthesis of polysubstituted furans utilizes Sn(II) and Cu(I)-catalyzed addition/oxidative cyclization of alkynoates and 1,3-dicarbonyl compounds, facilitated by the presence of 2,3-dichloro-5,6-dicyanobenzoquinone.
W. Liu, H. Jiang, M. Zhang, C. Qi, J. Org. Chem., 2010, 75, 966-968.
https://doi.org/10.1021/jo902375k
A solvent-free, ball-milling-assisted one-pot reaction of amines with alkyne esters and chalcones, promoted by the combination of I2 and PhI(OAc)2, efficiently yields a range of polysubstituted trans-2,3-dihydropyrroles in good quantities. Subsequent oxidation with DDQ provides the corresponding pyrroles.
H. Xu, H.-W. Liu, K. Chen, G.-W. Wang, J. Org. Chem., 2018, 83, 6035-6049.
https://doi.org/10.1021/acs.joc.8b00665
A regioselective hydroamination of alkynes with N-silylamine, catalyzed by a bis(amidate)bis(amido)titanium(IV) precatalyst, followed by the addition of α,β-unsaturated carbonyls to the crude reaction mixture and subsequent oxidation, yields 47 examples of pyridines with various substitution patterns, including pharmaceutically significant 2,4,5-trisubstituted pyridines, in good yields.
E. K. J. Lui, D. Hergesell, L. L. Schafer, Org. Lett., 2018, 20, 6663-6667.
https://doi.org/10.1021/acs.orglett.8b02703
The use of DDQ as an oxidant facilitates an efficient metal-free C-H amination of N-Ts-2-alkenylanilines, yielding a wide variety of substituted indoles. This straightforward and robust protocol avoids the need for costly transition-metal catalysts and displays a wide substrate scope. A proposed mechanism involves the formation of a radical cation through single electron transfer (SET) and a migratory process via a phenonium ion intermediate.
Y. H. Jang, S. W. Youn, Org. Lett., 2014, 16, 3720-3723.
https://doi.org/10.1021/ol5015398
High yields of various benzothiazoles were achieved through the intramolecular cyclization of thioformanilides, catalyzed by DDQ (2,3-dichloro-5,6-dicyanobenzoquinone) in dichloromethane at room temperature.
D. S. Bose, M. Idrees, B. Srikanth, Synthesis, 2007, 819-823.
https://doi.org/10.1055/s-2007-965929
A transition-metal-free method, utilizing DDQ as a mediator, allows for the intramolecular S-arylation of o-halobenzothiaoureas to produce 2-aminobenzothiazole derivatives. These reactions occur at room temperature without the need for a base, yielding very good product quantities.
R. Wang, W.-j. Yang, L. Yue, W. Pan, H.-y. Zeng, Synlett, 2012, 23, 1643-1648.
https://doi.org/10.1055/s-0031-1291159
Cobalt-catalyzed neutral Diels-Alder reactions of dienes derived from aldehydes with terminal and internal alkynes, followed by DDQ oxidation of the resulting dihydroaromatic intermediates, result in regiochemically enriched biphenyl, terphenyl, and silyl-functionalized benzene derivatives in yields ranging from good to excellent.
G. Hilt, M. Danz, Synthesis, 2008, 2257-2263.
https://doi.org/10.1055/s-2008-1078450
Cobalt-catalyzed Diels-Alder reactions of alkynyl pinacol boronic esters with diverse dienes produce cycloadducts with excellent regioselectivity. A seamless one-pot process, incorporating the Diels-Alder reaction, Suzuki coupling, and DDQ oxidation, was successfully executed without isolating the intermediates.
G. Hilt, K. I. Smolko, Angew. Chem. Int. Ed., 2003, 42, 2795-2797.
https://doi.org/10.1002/anie.200351404
Using [bis(trifluoroacetoxy)iodo]benzene as a stoichiometric oxidant and 2,3-dichloro-5,6-dicyanobenzoquinone as an organocatalyst, a straightforward oxidation of isochromans is achieved. Subsequent reaction with Grignard reagents or amides yields the corresponding isochroman derivatives.
W. Muramatsu, K. Nakano, Org. Lett., 2014, 16, 2042-2045.
https://doi.org/10.1021/ol5006399
A straightforward oxidative coupling of α-carbonyl radicals with DDQ (2,3-dichloro-5,6-dicyanobenzoquinone) allows for the synthesis of 2,3-dicyanofurans and thiophenes from easily accessible β-diketones, simple ketones, and β-keto thioamides, yielding high product quantities. Mechanistic studies suggest that this transformation may involve a radical process and water-facilitated C-C bond cleavage.
Z.-L. Wang, H.-L. Li, L.-S. Ge, X.-L. An, Z.-G. Zhang, X. Luo, J. S. Fossey, W.-P. Deng, J. Org. Chem., 2014, 79, 1156-1165.
https://doi.org/10.1021/jo4026034
A streamlined one-pot synthesis of highly functionalized pyridines involves the formal insertion of rhodium vinylcarbenoids, derived from diazo compounds, across the N-O bond of isoxazoles. Upon heating, these insertion products undergo a rearrangement to produce 1,4-dihydropyridines. Subsequent DDQ oxidation yields the corresponding pyridines in good quantities.
J. R. Manning, H. M. L. Davies, J. Am. Chem. Soc., 2008, 130, 8602-8603.
https://doi.org/10.1021/ja803139k
A copper-catalyzed tandem reaction of 2-aminobenzamides with tertiary amines yields quinazolinone derivatives under standard conditions. This process is applicable to a wide range of substrates, resulting in the formation of the corresponding quinazolinone derivatives in good yields.
W. Xu, X.-R. Zhu, P.-C. Qian, X.-G. Zhang, C.-L. Deng, Synlett, 2016, 27, 2851-2857.
https://doi.org/10.1055/s-0036-1588881
Triflic anhydride, in the presence of N,N-dimethyl trifluoroacetamide (DTA), promotes a straightforward and effective intramolecular cyclization of easily accessible N-aryl cinnamides under gentle conditions, yielding polysubstituted quinolin-2(1H)-ones.
Q. Zhang, J. Yuan, M. Yu, R. Zhang, Y. Liang, P. Huang, D. Dong, Synthesis, 2017, 49, 4996-5002.
https://doi.org/10.1055/s-0036-1590821
A visible-light photocatalyzed aerobic oxidative lactonization of arene C(sp2)-H bonds, in the presence of DDQ (2,3-dichloro-5,6-dicyanobenzoquinone) and tert-butyl nitrite, efficiently produces benzocoumarin derivatives in good yields. This environmentally friendly process features mild reaction conditions, the use of a green oxidant, and metal-free catalysis.
Y. Wang, S. Wang, B. Chen, M. Li, X. Hu, B. Hu, L. Jin, N. Sun, Z. Shen, Synlett, 2020, 31, 261-266.
https://doi.org/10.1055/s-0039-1691537
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/ddq-2,3-dichloro-5,6-dicyanobenzoquinone.shtm
Aladdin:https://www.aladdinsci.com





















