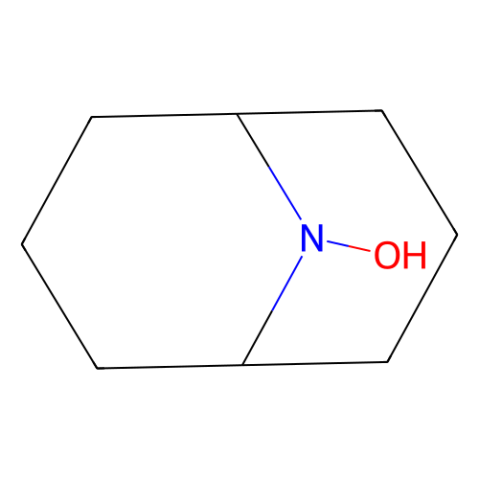
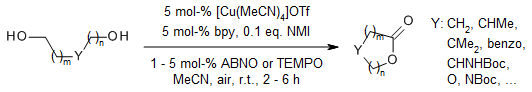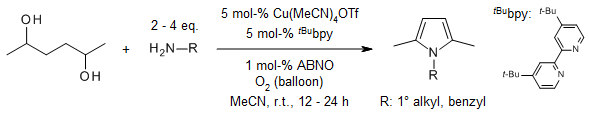9-Azabicyclo[3.3.1]nonane N-Oxyl, ABNO
Product Manager:Nick Wilde

9-Azabicyclo[3.3.1]nonane N-Oxyl (ABNO) is a less hindered nitroxyl radical, that exhibits an enhanced reactivity compared with TEMPO.
Recent Literature
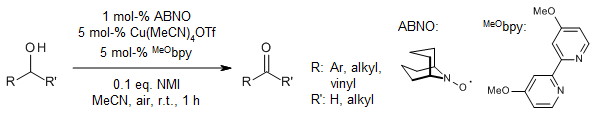
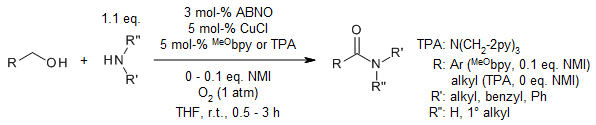
Cu/TEMPO catalyst systems show reduced reactivity in aerobic oxidation of aliphatic and secondary alcohols. A catalyst system consisting of (MeO-bpy)CuOTf and ABNO mediates aerobic oxidation of primary, secondary allylic, benzylic, and aliphatic alcohols with nearly equal efficiency. The catalyst exhibits broad functional group compatibility, and most reactions are complete within 1 h at room temperature using ambient air as oxidant.
J.E. Steves, S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 15742-15745. https://doi.org/10.1021/ja409241h
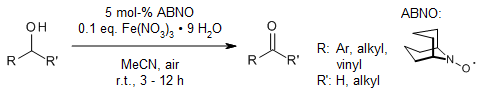
The combination of Fe(NO3)3·9H2O and 9-azabicyclo[3.3.1]nonan-N-oxyl enables an efficient aerobic oxidation of a broad range of primary and secondary alcohols to the corresponding aldehydes and ketones at room temperature with ambient air as the oxidant.
L. Wang, S. Shang, G. Li, L. Ren, Y. Lv, S. Gao, J. Org. Chem., 2016, 81, 2189-2193. https://doi.org/10.1021/acs.joc.6b00009
A modular Cu/ABNO catalyst system enables efficient aerobic oxidative coupling of alcohols and amines to amides. All four permutations of benzylic/aliphatic alcohols and primary/secondary amines are viable in this reaction, enabling broad access to secondary and tertiary amides with excellent functional group compatibility within short reaction time at rt.
S.L. Zultanski, J. Zhao, S. S. Stahl, J. Am. Chem. Soc., 2016, 138, 6416-6419. https://doi.org/10.1021/jacs.6b03931
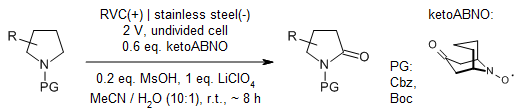
A selective electrochemical aminoxyl-mediated Shono-type oxidation of pyrrolidines provides pyrrolidinones with high selectivity and functional group compatibility.
N.R. Deprez, D. J. Clausen, J.-X. Yan, F. Peng, S. Zhang, J. Kong, Y. Bai, Org. Lett., 2021, 23, 8834-8837.
https://doi.org/10.1021/acs.orglett.1c03338
Cu/nitroxyl catalysts promote a highly efficient and selective aerobic oxidative lactonization of diols under mild reaction conditions using ambient air as the oxidant. A Cu/ABNO catalyst system shows excellent reactivity with symmetrical diols and hindered unsymmetrical diols, whereas a Cu/TEMPO catalyst system displays excellent chemo-and regioselectivity for the oxidation of less hindered unsymmetrical diols.
X. Xie, S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 3767-3770.
https://doi.org/10.1021/jacs.5b01036
A Cu/ABNO-catalyzed aerobic oxidative coupling of diols and a broad range of primary amines provides N-substituted pyrroles. The reaction proceeds at room temperature with an O2 balloon as the oxidant using commercially available materials as the substrates and catalysts. The catalyst system offers a good tolerance to sensitive functional groups.
W. Fu, L. Zhu, S. Tan, Z. Zhao, X. Yu, L. Wang, J. Org. Chem., 2022, 87, 13389-13395. https://doi.org/10.1021/acs.joc.2c01646
Quoted from:
https://www.organic-chemistry.org/chemicals/oxidations/9-azabicyclo[3.3.1]nonane-N-oxyl-abno.shtm
Aladdin:https://www.aladdinsci.com