Applications of Fullerenes in Bioscience and Optoelectronics
Introduction
Twenty-five years ago, it was discovered that carbon vapor preferentially condenses into an extremely stable cluster of 60 carbon atoms, assuming that these 60 atoms are arranged to form the vertices of a truncated icosahedron (shaped like a soccer ball) [1]. The carbon cluster was named Buckminsterfullerene, after the architect of the geodesic dome-based building structure, Fuller. Five years later, through a synthesis method using milligram quantities of Buckminster fullerenes, it was discovered that not only the C60 clusters are not only stable in air, but also soluble in aromatic solvents [2] . Now that it is correctly considered a molecule rather than a metastable cluster, it is clear that C60 is not the only " fullerene " , but a whole family of stable, soluble, all-carbon carbon molecules with C70 (Product No.: F107608 ) , C76 (Product No.: F281429 ), C78 (Product No.: F281430 ), C84 (Product No.: F281427), and even some molecules have more than 100 atoms (and no hydrogen atoms). In nature, fullerenes have been detected in interstellar dust and meteorite impacts. Over time, the technology of synthesizing fullerenes from carbon steam has developed from the gram scale to the kilogram scale, and finally the technology of synthesizing fullerenes in a soot flame enables the production of fullerenes to reach the ton scale [3]. Therefore, once by Eiji’s question that Prof. Osawa describes as "chicken-egg or chicken-and-egg" - that fullerenes have no commercial application because there is no large-scale production and no large-scale production because there is no commercial application - has been solved.
The development of fullerenes for various commercial applications is currently in a stage of rapid development. In select markets, consumers can buy sporting goods such as badminton rackets containing polymer matrix composites containing fullerenes, as well as cosmetics such as vitamin C 60 skin cream. Fullerene materials have been widely used, for example, filled with other forms of carbon, including catalyst supports, battery anodes, and proton transport membranes, among others. However, the most widely known and promising advances in fullerene research to date have been in the fields of organic electronics and biological sciences.
Applications of fullerenes in optoelectronics
During electron transport, fullerenes have a low probability of carrier recombination, which makes them one of the most useful electron acceptor molecules in organic electronics [4]. At the same time, they are also n-type semiconductors (bandgap = 2.3 eV), which makes them ideal coupling materials for many excellent p-type organic semiconductors. The nearly spherical structure enables electron transfer to occur in any spatial direction without preference, and their electron mobility (µe=0.1cm2 /Vs) is well suited for organic materials. C60 or C70 can be used to make transistors with excellent performance in an inert environment. Additionally, many C60 anionic (“fullerate”) salts are superconductors with appreciable transition temperatures (~30 K).
Fullerenes are also widely used in the field of organic photovoltaics (OPVs). For example, significant progress has been made in the power conversion efficiency of devices and the development of more stable plastic solar cell architectures [5]. Fullerenes act as electron acceptors in almost all reported high-performance devices (Fig. 1). C60 is the standard for vacuum-deposited OPVs, and the more soluble PCBM (Product No.: B405184) is the standard for solution-deposited OPVs. Further performance improvements can usually be achieved with a PC70BM (Product No.: P400139). The energy bands of fullerenes are moderately aligned with the energy bands of electron-donating molecules, which facilitates exciton dissociation and charge transfer. It is expected that research related to this advanced technology will develop rapidly in the near future.
of fullerenes in the field of biological sciences
fullerenes add an electron and then remove that electron consumes little energy and is at the heart of the main biological application of fullerenes: as antioxidants. Certain water-soluble fullerenes can act as analogs of naturally occurring superoxide dismutase, catalyzing the removal of potentially harmful oxides. Their antioxidant behavior, or possibly the anti-proliferative consequence of scavenging free radicals involved in the cell cycle, prolongs the lifespan of older mice and also improves their age cognition [6]. Therefore, water-soluble fullerenes, such as C60 pyrrolidine triacid (Product No.: N404946), are used to treat various diseases involving excess reactive oxygen species.
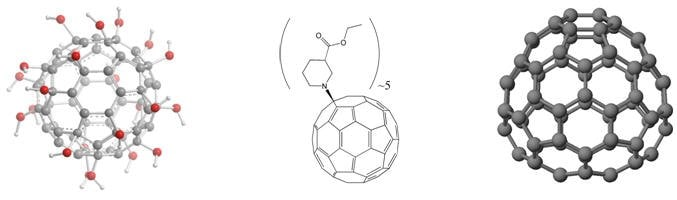
Fullerenes are also being developed for drug delivery. Researchers have developed a number of ways to form covalent bonds with fullerenes. Using these methods, various therapeutic agents can be covalently attached to fullerenes, allowing their pharmacokinetic properties to be tailored [7]. While many nanomaterials are being developed for the same purpose, the advantages of fullerenes are still evident, for example, their compact size (~1 nm diameter, as shown in Figure 2) in enhancing solubility and avoiding aggregation during transport Better than nanoparticles (10-100nm diameter). Furthermore, C60 is a special pure molecule, while covalently bound derivatives can be easily characterized using standard analytical chemistry techniques.

Figure 2 Hypothetical fullerene antibody constructed from C82 carboxy metallo fullerene and chimeric B72.3 Fab ' antibody fragment body conjugates.
The cavity in the middle of the carbon cage is another inherent and unique property of fullerenes. This space can hold atoms, or groups of atoms of at most a few atoms. Once atoms are inside fullerenes, only high-energy events, such as nuclear recoil after alpha decay, can let them escape. Therefore, these "endohedral fullerenes," fullerenes with one or more atoms inside, are being developed as imaging agents. Imaging is still enabled by encapsulated atoms, but biodistribution is now determined by fullerenes and covalent bonds attached to their exterior. Magnetic resonance imaging (MRI) contrast agents are a prime example of the application of fullerene imaging agents, and fullerene transport of radioisotopes is also being explored. Many fullerene derivatives have also been developed as sensitizers for photodynamic therapy, as well as aggregates for DNA transfection.
Although preliminary reports of the significant toxicity of aqueous suspensions of C60 aggregates ("nano-C60") have attracted widespread attention in the media. But subsequent investigations revealed that the toxicity was due to a by-product of the process of forming an aqueous suspension, rather than the fullerenes themselves [9]. However, fullerene derivatives containing specific chemical groups can disrupt cell walls and are used as antibacterial and antiviral agents.
Small gap fullerenes
So far, all commercially available fullerenes have been recovered from non- fullerene carbon substrates by solvent extraction. However, the solvent extraction process is relatively inefficient because many fullerenes will polymerize to an insoluble state. Polymerization can be mediated by oxygen, but can also occur spontaneously. Fullerenes with a small energy difference between the electron-filled and unfilled states of a single fullerene molecule (“small-interstitial fullerenes”) are particularly prone to this spontaneous polymerization [12], and therefore cannot be extracted by solvents. to recycle. As the "cage" size increases, the percentage of "small gap" isomers for a certain "cage" size increase dramatically. Therefore, the solvent-extracted fullerenes contain few large- gap fullerenes, while small-gap fullerenes (SGFs) are abundant [11]. Although SGFs are relatively abundant, they are often disposed of as waste along with non- fullerene carbon substrates.
Figure 3 compares the distribution of fullerenes in raw combustion soot with that of SGF fullerenes and conventional solvent-extracted fullerenes. Mass spectra were generated by time-of-flight mass spectrometry (TOF-MS) using a 355 nm laser pulse for desorption of fullerenes and a 118 nm laser pulse for fullerene ionization. Since all fullerenes are ionized by a single 118 nm photon, the described distribution of fullerenes in the gas phase is accurate, although not all fullerenes may be desorbed with the same efficiency. Analysis of the peak heights indicated that 60% of the fullerenes present were greater than C70 and 50 % of the fullerenes present were greater than C84. The rest of C60 and C70 appear to be bound to the polymer and thus not extracted by the solvent. SGFs are very abundant in fullerene "cage" sizes and isomers, which are not present in traditional solvent-extracted fullerenes, such as C74.

Figure 3 Top: Mass distribution of small-gap fullerene products. Bottom: distribution of fullerenes recovered by traditional solvent extraction
SGFs can be used both as fullerene materials and as fullerene derivatives through covalent chemical modification. By hiding residual hydrocarbon impurities into the carbon after receiving or thermal treatment, SGFs form a new class of nanostructured carbon materials that can be used as adsorbents, catalyst supports, or additives for polymer matrices. After reduction into a suitable solvent, the fullerene anions are discrete soluble molecules, and thin films of SGFs can be plated onto many substrates to form novel carbon coatings (Figure 4). For example, the bulk conductivity of pressed SGFs pellets is about 10 -4 S/cm.

Fig.4 SGFs film electroplated on titanium foil
Although the chemistry of SGFs is still in the exploratory stage, some known reactions of C60 also derivatize SGF, resulting in soluble products [13]. For example, water-soluble polyhydroxy SGFs can be produced, which can be used as water-soluble antioxidant/antiproliferative agents. Alternatively, organic-soluble SGF esters such as those shown in Figure 5 can also be produced, which may find applications as n-type materials in solution-processable organic electronics.
Figure 5 Left: the structure of water-soluble poly-hydroxy fullerene SGF; middle: the structure of organic soluble SGF esters. Right: C 74 block diagram
The SGF presented here represents the third carbon material after graphite and diamond, and given the technical importance of the first two materials, the future of SGF is also predicted to be very bright.
References
1.Kroto HW, Heath JR, O?Brien SC, Curl RF, Smalley RE. 1985. C60: Buckminsterfullerene. Nature. 318(6042):162-163. http://dx.doi.org/10.1038/318162a0
2.Krätschmer W, Lamb LD, Fostiropoulos K, Huffman DR. 1990. Solid C60: a new form of carbon. Nature. 347(6291):354-358. http://dx.doi.org/10.1038/347354a0
3.Takehara H, Fujiwara M, Arikawa M, Diener MD, Alford JM. 2005. Experimental study of industrial scale fullerene production by combustion synthesis. Carbon. 43(2):311-319. http://dx.doi.org/10.1016/j.carbon.2004.09.017
4.Imahori H, Fukuzumi S. 2004. Porphyrin- and Fullerene-Based Molecular Photovoltaic Devices. Adv. Funct. Mater. 14(6):525-536. http://dx.doi.org/10.1002/adfm.200305172
5.Bredas J, Durrant JR. 2009. Organic Photovoltaics. Acc. Chem. Res. 42(11):1689-1690. http://dx.doi.org/10.1021/ar900238j
6.Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. 2008. A carboxy fullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiology of Aging. 29(1):117-128. http://dx.doi.org/10.1016/j.neurobiolaging.2006.09.014
7.Zakharian TY, Seryshev A, Sitharaman B, Gilbert BE, Knight V, Wilson LJ. 2005. A Fullerene? Paclitaxel Chemotherapeutic: Synthesis, Characterization, and Study of Biological Activity in Tissue Culture. J. Am. Chem. Soc. 127(36):12508-12509. http://dx.doi.org/10.1021/ja0546525
8.Bolskar RD. 2008. Gado fullerene MRI contrast agents. Nanomedicine. 3(2):201-213. http://dx.doi.org/10.2217/17435889.3.2.201
9.Henry TB, Menn F, Fleming JT, Wilgus J, Compton RN, Sayler GS. 2007. Attributing Effects of Aqueous C 60 Nano-Aggregates to Tetrahydrofuran Decomposition Products in Larval Zebrafish by Assessment of Gene Expression. Environmental Health Perspectives. 115(7):1059-1065. http://dx.doi.org/10.1289/ehp.9757
10.Tsao N, Luh T, Chou C, Chang T, Wu J, Liu C, Lei H. 2002. In vitro action of carboxy fullerene. Journal of Antimicrobial Chemotherapy. 49(4):641-649. http://dx.doi.org/10.1093/jac/49.4.641
11.Diener MD, Alford JM. 1998. Isolation and properties of small-bandgap fullerenes. Nature. 393(6686):668-671. http://dx.doi.org/10.1038/31435
12.Okada S, Saito S. 2000. Stable polymers of C74 and C78 fullerenes. Chemical Physics Letters. 321(1-2):156-162. http://dx.doi.org/10.1016/s0009-2614(00)00292-x
13.Goryunkov AA, Markov VY, Ioffe IN, Bolskar RD, Diener MD, Kuvychko IV, Strauss SH, Boltalina OV. 2004. C74F38: An Exohedral Derivative of a Small-Bandgap Fullerene withD3 Symmetry. Angew. Chem. Int. Ed. 43(8):997-1000. http://dx.doi.org/10.1002/anie.200352960