Aryl fluorination

Introduction
Aryl fluoride building blocks refer to a series of aromatic compounds with one or several fluorine atoms directly connected to the aromatic ring skeleton. When fluorine is introduced into aromatics, it can provide many excellent properties. First of all, fluorinated aromatic hydrocarbons are more lipophilic than non-fluorinated aromatic hydrocarbons, which can be used in drug development to improve the fat solubility of drug molecules and enhance the penetration of fluorinated drug molecules into membranes and tissues in the organism, thus improving the absorption and transmission speed in the organism.Furthermore, in pharmaceutical chemistry, fluorine is sometimes used as an electronic isotope of hydrogen (the spatial parameters of hydrogen and fluorine are similar, and the van der Waals radii are 1.2Å and 1.35Å , respectively).In addition, fluorinated compounds can also be strategically used as transition state inhibitors.
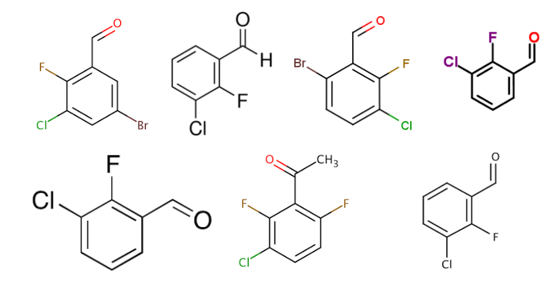
Fig.1. Aryl fluorinated building blocks
Due to the nature of fluorine itself, any reaction to generate C-F bond is a major challenge no matter what method is used. Fluoride anions can form strong hydrogen bonds with various hydrogen bond donors such as water, alcohols, amines and amides due to their electronegativity and small ionic radius (1.33Å). The high solubility of fluoride ions in water medium will lead to a closely combined water molecule hydration layer around it. Therefore, in the presence of hydrogen bond donors, fluoride usually has only weak nucleophilicity, which limits the formation of C-F bonds through nucleophilic substitution reaction.
Approaches to Arene Fluorination
Traditional Nucleophilic Arene Fluorination Reactions
A major challenge with respect to the traditional fluorination of nucleophilic aromatic hydrocarbons is the limitation of relatively simple substrates, which is caused by harsh reaction conditions and the strong toxicity of nucleophilic fluorides. In 1927, Balz and Schiemann developed the nucleophilic fluorination reaction of aromatic hydrocarbons by decomposing primary aromatic amines into aryl fluorides1-2 via the diazo intermediate of tetrafluoroborate through overheating (Fig. 2).
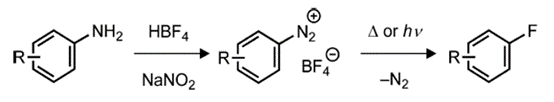
Fig.2. Nucleophilic fluorination of aryl diazonium salts
This reaction is similar to Sandmeyer reaction in concept, which converts diazonium salt into other aryl halides (ArCl, ArBr) 3. However, although the Sandmeyer reaction involves copper reagents/catalysts and free radical intermediates, the thermal decomposition of tetrafluoroborate diazo proceeds without a co catalyst to generate highly unstable aryl cations (Ar+), thereby extracting F− from BF4− to obtain fluoroaromatic hydrocarbons (ArF) and boron trifluoride as a by-product.
Nucleophilic fluorination reactions
Nucleophilic fluorination is a simple and effective method for nucleophilic fluorination of aromatic hydrocarbons, which excludes water and other hydrogen bond donors that may weaken the nucleophilic property of fluoride. Tetrabutylammonium fluoride (TBAF) is a commercially available nucleophilic reagent that is easily soluble in organic solvents and can be used as trihydrate (Fig. 3).

Fig.3. nucleophilic arene fluorination using TBAF
Nucleophilic deoxyfluorination of phenols
In addition to aryl halides, phenol is a more reactive and readily available substrate for the synthesis of aryl fluoride. For example, catechol can be deoxidized and fluorinated first, and then reduced with sodium borohydride (Fig. 4).
Fig.4. Deoxyfluorination of catechols via a one-pot reaction
Electrophilic fluorination reactions
The method for complementing the aryl fluorination is to use aryl nucleophilic reagents and electrophilic fluorinating reagents. N-fluoro reagent can be used as the source of fluoronium cations (F+) in form. The commonly used electrophilic fluorination reagent is shown in Figure 5.
Fig.5. Commonly used electrophilic fluorinating reagents
Among them, Selectfluor (N-Fluoro-N'-(chloromethyl)triethylenediamine Bis(tetrafluoroborate) or F-TEDA) is a user friendly, mild, air and water stable, nonvolatile electrophilic fluorinating reagent. Selectfluor reagent can introduce fluorine into organic substrate in one step, and has very wide reactivity4. In addition, these reactions showed good regioselectivity.
A powerful and noncytotoxic nucleoside inhibitor of Hepatitis C virus RNA replication was synthesized with Selectfluor fluorination reagent (Fig. 6). Compared with the parent 2 '- C-methyladenosine, this riboside shows significant enzymatic stability 5.
Fig. 6 Electrophilic Fluorination Reagent Using Selectfluor
Applications
Aryl fluorinated building blocks are widely used as intermediates or drug molecules of synthetic drugs, in which fluorine substitution can improve the efficacy and selectivity to the target by influencing pKa, adjusting conformation, hydrophobic interaction and lipophilicity or the superposition of these properties. In addition, the aryl fluoride block can also be used in pesticides, plastics and molecules related to liquid crystal technology.
Reference
1.Balz, Günther; Schiemann, Günther (1927). "Über aromatische Fluorverbindungen, I.: Ein neues Verfahren zu ihrer Darstellung" [Aromatic fluorine compounds. I. A new method for their preparation.]. Chemische Berichte (in German). 60 (5): 1186–1190. https://doi.org/10.1002/cber.19270600539
2.Furuya, Takeru; Klein, Johannes E. M. N.; Ritter, Tobias (2010). "C–F Bond Formation for the Synthesis of Aryl Fluorides". Synthesis. 2010 (11): 1804–1821. https://doi.org/10.1055/s-0029-1218742
3.Swain, C. G.; Rogers, R. J. (1975). "Mechanism of formation of aryl fluorides from arenediazonium fluoborates". J. Am. Chem. Soc. 97 (4): 799–800. https://doi.org/10.1021/ja00837a019
4.Singh, R. P. , Shreeve, J. M.. 2004. For a review of recent highlights: Acc. Chem. Res..37, 31.
5.Eldrup AB, Prhavc M, Brooks J, Bhat B, Prakash TP, Song Q, Bera S, Bhat N, Dande P, Cook PD, et al. 2004. Structure?Activity Relationship of Heterobase-Modified 2’-C-Methyl Ribonucleosides as Inhibitors of Hepatitis C Virus RNA Replication. J. Med. Chem.. 47(21):5284-5297.
https://doi.org/10.1021/jm040068f


