Basic Concepts about Catalysts

Transition-State Theory1
Transition-state theory, also called activated-complex theory or theory of absolute reaction rates, treatment of chemical reactions and other processes that regards them as proceeding by a continuous change in the relative positions and potential energies of the constituent atoms and molecules. On the reaction path between the initial and final arrangements of atoms or molecules, there exists an intermediate configuration at which the potential energy has a maximum value. The configuration corresponding to this maximum is known as the activated complex, and its state is referred to as the transition state. The difference between the energies of the transition and the initial states is closely related to the experimental activation energy for the reaction; it represents the minimum energy that a reacting or flowing system must acquire for the transformation to take place. In transition-state theory, the activated complex is considered to have been formed in a state of equilibrium with the atoms or molecules in the initial state, and therefore its statistical and thermodynamic properties can be specified. The rate at which the final state is attained is determined by the number of activated complexes formed and the frequency with which they go over to the final state. These quantities may be calculated for simple systems by using statistical-mechanical principles. In this way the rate constant of a chemical or physical process may be expressed in terms of atomic and molecular dimensions, atomic masses, and interatomic or intermolecular forces. Transition-state theory can also be formulated in thermodynamic terms.
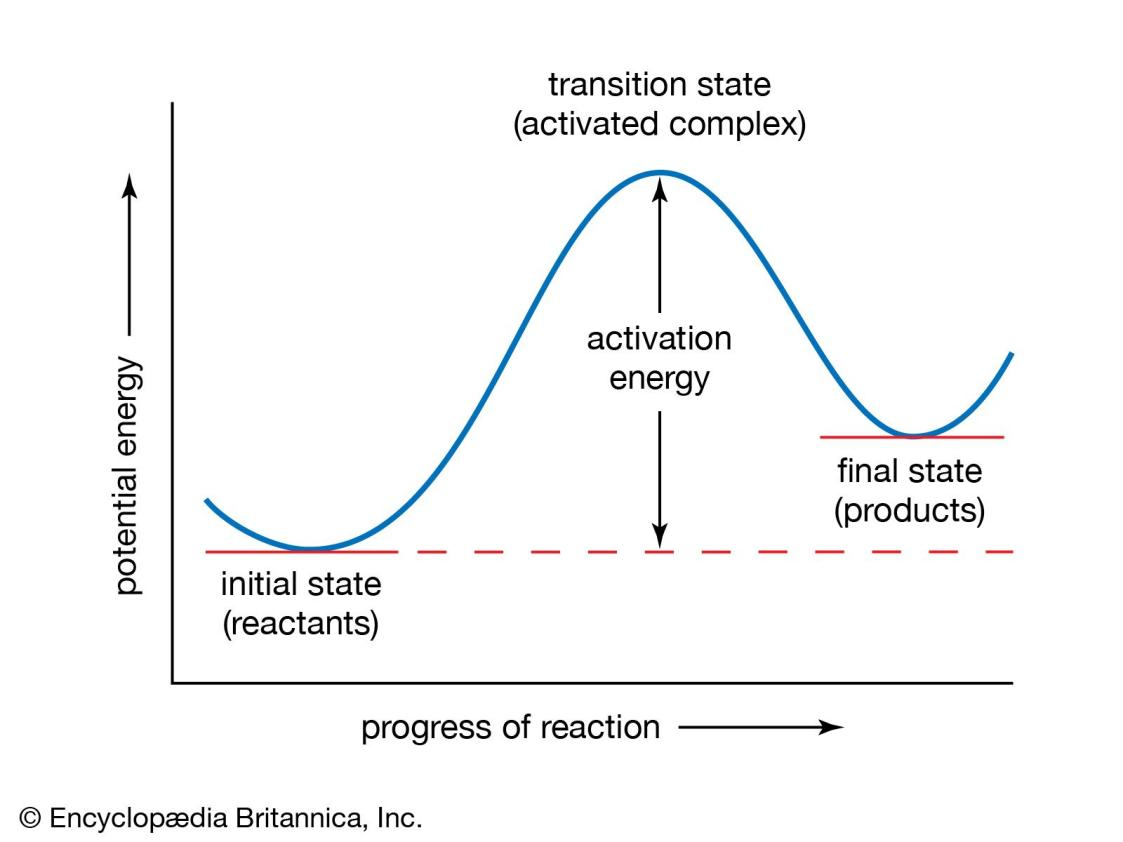
Figure 1. Potential-energy curve.
The activation energy represents the minimum amount of energy required to transform reactants into products in a chemical reaction. The value of the activation energy is equivalent to the difference in potential energy between particles in an intermediate configuration (known as the transition state, or activated complex) and particles of reactants in their initial state. The activation energy thus can be visualized as a barrier that must be overcome by reactants before products can be formed.
Heat of Reaction2
Heat of reaction, also called enthalpy of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the same temperature. If the pressure in the vessel containing the reacting system is kept at a constant value, the measured heat of reaction also represents the change in the thermodynamic quantity called enthalpy, or heat content, accompanying the process—i.e., the difference between the enthalpy of the substances present at the end of the reaction and the enthalpy of the substances present at the start of the reaction. Thus, the heat of reaction determined at constant pressure is also designated the enthalpy of reaction, represented by the symbol ΔH. If the heat of reaction is positive, the reaction is said to be endothermic; if negative, exothermic.
The prediction and measurement of the heat effects that accompany chemical changes are important to the understanding and use of chemical reactions. If the vessel containing the reacting system is so insulated that no heat flows into or out of the system (adiabatic condition), the heat effect that accompanies the transformation may be manifested by an increase or a decrease in temperature, as the case may be, of the substances present. Accurate values of heats of reactions are necessary for the proper design of equipment for use in chemical processes.
Because it is not practical to make a heat measurement for every reaction that occurs and because for certain reactions such a measurement may not even be feasible, it is customary to estimate heats of reactions from suitable combinations of compiled standard thermal data. These data usually take the form of standard heats of formation and heats of combustion. The standard heat of formation is defined as the amount of heat absorbed or evolved at 25 °C (77 °F ) and at one atmosphere pressure when one mole of a compound is formed from its constituent elements, each substance being in its normal physical state (gas, liquid, or solid). The heat of formation of an element is arbitrarily assigned a value of zero. The standard heat of combustion is similarly defined as the amount of heat evolved at 25 °C and at one atmosphere pressure when one mole of a substance is burned in excess oxygen. The method of calculating heats of reactions from measured values of heats of formation and combustion is based on the principle known as Hess’s law of heat summation.
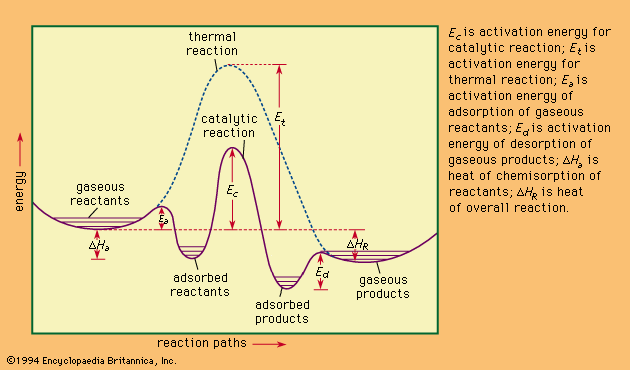
Figure 2. Energy profiles for catalytic and thermal (noncatalytic) reactions.
Catalyst3
Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions.
Most solid catalysts are metals or the oxides, sulfides, and halides of metallic elements and of the semimetallic elements boron, aluminum, and silicon. Gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; solid catalysts are commonly dispersed in other substances known as catalyst supports.
In general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more readily with each other or with another reactant, to form the desired end product. During the reaction between the chemical intermediates and the reactants, the catalyst is regenerated. The modes of reactions between the catalysts and the reactants vary widely and in solid catalysts are often complex. Typical of these reactions are acid–base reactions, oxidation–reduction reactions, formation of coordination complexes, and formation of free radicals. With solid catalysts the reaction mechanism is strongly influenced by surface properties and electronic or crystal structures. Certain solid catalysts, called polyfunctional catalysts, are capable of more than one mode of interaction with the reactants; bifunctional catalysts are used extensively for reforming reactions in the petroleum industry.
Catalyzed reactions form the basis of many industrial chemical processes. Catalyst manufacture is itself a rapidly growing industrial process.
Catalytic processes and their catalysts | |
process | catalyst |
ammonia synthesis | iron |
sulfuric acid manufacture | nitrogen(II) oxide, platinum |
cracking of petroleum | zeolites |
hydrogenation of unsaturated hydrocarbons | nickel, platinum, or palladium |
oxidation of hydrocarbons in automobile exhausts | copper(II) oxide, vanadium(V) oxide, platinum, palladium |
isomerization of n-butane to isobutane | aluminum chloride, hydrogen chloride |

Figure 3. Ziegler-Natta polymerization of ethylene.
Ethylene gas is pumped under pressure into a reaction vessel, where it polymerizes under the influence of a Ziegler-Natta catalyst in the presence of a solvent. A slurry of polyethylene, unreacted ethylene monomer, catalyst, and solvent exit the reactor. Unreacted ethylene is separated and returned to the reactor, while the catalyst is neutralized by an alcohol wash and filtered out. Excess solvent is recovered from a hot water bath and recycled, and a dryer dehydrates the wet polyethylene to its final powder form.
Chemical Intermediate4
Chemical intermediate, any chemical substance produced during the conversion of some reactant to a product. Most synthetic processes involve transformation of some readily available and often inexpensive substance to some desired product through a succession of steps. All the substances generated by one step and used for the succeeding step are considered intermediates.
Apart from substances that can be recovered as products if the reaction is stopped at the point of generation of the intermediate, unstable molecules, some chemical substances are either known or hypothesized to be intermediate, even if they have not yet been isolated. Among the classes of generally unstable intermediates that are well studied are free radicals, carbenes, carbonium ions, and carbanions. These intermediates are highly reactive fragments of molecules that ordinarily remain uncombined for only very short periods of time.
References
1. Laidler, Keith J.. "transition-state theory". Encyclopedia Britannica, 23 Sep. 2019, https://www.britannica.com/science/transition-state-theory.
2. Britannica, The Editors of Encyclopaedia. "heat of reaction". Encyclopedia Britannica, 25 Aug. 2022, https://www.britannica.com/science/heat-of-reaction.
3. Britannica, The Editors of Encyclopaedia. "catalyst". Encyclopedia Britannica, 30 Oct. 2022, https://www.britannica.com/science/catalyst.
4. Britannica, The Editors of Encyclopaedia. "chemical intermediate". Encyclopedia Britannica, 20 Jul. 1998, https://www.britannica.com/science/chemical-intermediate.
