Biginelli Reaction
Introduction
The Biginelli Reaction (Figure 1) is an acid-catalyzed three-component reaction between an aromatic aldehyde, a β-keto ester, and a urea (e.g., urea, thiourea) to produce a tetrahydropyrimidinone with potential pharmaceutical applications.

Fig. 1. Biginelli reaction
This reaction was first reported by the Italian chemist Pietro Biginelli in 1891 and has been of increasing interest due to the inextricable structural relationship of the end product with the drug intermediate dihydropyrimidine. 1 Such compounds are known to possess biological activities such as antiviral, antitumor, antibacterial, anti-inflammatory and, more recently, in antihypertensive drugs. 2
Mechanism
The Biginelli reaction begins with the protonation of an aromatic aldehyde by an acid, followed by the attack of an amine by a urea. The proton transfer then produces a protonated alcohol which departs as water to form an N-acyl imino ion intermediate. The intermediate is then attacked by the enol form of the β-keto ester. Another amine group reacts with the carbonyl group to produce a cyclic intermediate. The cyclized pyrimidinone end product is produced by a proton transfer step, water departure and deprotonation. 3,4
Fig. 2. Biginelli reaction mechanism
Characteristics
1. The Biginelli reaction is usually carried out in alcoholic solvents with a small amount of acid as catalyst.
2. Where the catalysts are generally Lewis acids and Brönsted acids such as HCl, H2SO4, TsOH, TMSI, LiBr, InBr3, BF3-OEt2, FeCl3, Yb(OTf)3, Bi(OTf)3, VCl3, PPE.
3. More room for expansion of the structures of the three reaction components.
4. Aliphatic aldehydes, aromatic aldehydes or heterocyclic aromatic aldehydes can be involved in this reaction, but the yield of aliphatic aldehydes and aromatic aldehydes with large spatial site resistance (such as o-position substituted aromatic aldehydes) is lower.
5. Various other β-keto esters and tertiary acetyl acetamides have been used in this reaction.
6. Monosubstituted urea and thiourea will only produce N-1 substituted dihydropyrimidinones by this reaction, and N-3 alkyl substituted products will hardly be produced.
7. N,N'-disubstituted urea will not react under standard Biginelli reaction conditions.
8. Asymmetric Biginelli reactions catalyzed by chiral phosphoric acid have been reported in the literature.
Advances in Biginelli Reaction
1.Atwal modified method: Atwal et al. used α,β-unsaturated carbonyl compounds to condense with protected urea or thiourea under near-neutral conditions to 1,4-dihydropyrimidine, and then deprotected to obtain the corresponding dihydropyrimidinone, generally in higher yields.
2. Shutalev modified method: α-tosyl-substituted urea or thiourea reacts with the enol salt of 1,3-dicarbonyl compound to obtain hexahydropyrimidine, which is more easily converted to dihydropyrimidinones and derivatives (DHPMs).
3. Solid-phase synthesis method: High-purity dihydropyrimidinones and derivatives were synthesized in high yields using the reaction of king resin urea derivatives or polyethylene glycol acetoacetate.
4.Biginelli reaction has been reported to be successfully carried out under microwave-assisted and solvent-free conditions.
Recent Research and Trends
1. The joint role of asymmetric counteranion-directed catalysis (ACDC) and ionic liquid effect (ILE) in enantiomer Biginelli multi-component reactions. 5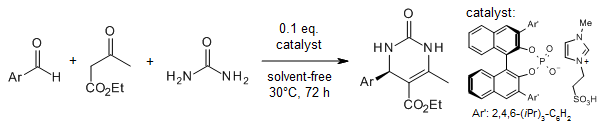
2. By using Yb(OTf)3 as a catalyst, the yield of one pot Biginelli reaction can be increased and the reaction time can be shortened under solvent-free reaction conditions. In addition, catalysts can be easily recovered and reused. It not only realizes atom economy, but also reduces dangerous pollution and realizes environment-friendly process.6
3.The improved Biginelli reaction of 1,3-dicarbonyl compounds, aldehydes and ureas catalyzed by indium chloride (III) provides an effective way to synthesize dihydropyrimidinones with high yield. This mild reaction can be carried out in one pot and tolerate various substitutions.7
4.New substrate for Biginelli ring condensation: β-Direct preparation of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)- ketones from ketone carboxylic acids. When TFA is used in the Biginelli reaction with electron rich and electron poor aldehydes in reflux dichloroethane, oxalic acid is an excellent substrate, providing functional groups that can be widely converted.8
Reference
1.Kappe, C.O. J. . 1997. Org. Chem. .(62):7201–7204.
2.Rafiee E, Jafari H. 2006. A practical and green approach towards synthesis of dihydropyrimidinones: Using heteropoly acids as efficient catalysts. Bioorganic & Medicinal Chemistry Letters. 16(9):2463-2466. https://doi.org/10.1016/j.bmcl.2006.01.087
3.Biginelli, P. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319.https://doi.org/10.1002/cber.189102401228
4.Biginelli, P. Ber. Dtsch. Chem. Ges. 1891, 24, 2962–2967.https://doi.org/10.1002/cber.189102402126
5.H.G.O. Alvim D.L.J. Pinheiro, V. H. Carvalho-Silva, M. Fioramonte, F. C. Gozzo, W.A.da Silva, G.W. Amarante, B.A.D. Neto, J. Org. Chem., 2018, 83, 12143-12153.https://doi.org/10.1021/acs.joc.8b02101
6.Y. Ma, C. Qian, L. Wang, M. Yang, J. Org. Chem., 2000, 65, 3864-3868. https://doi.org/10.1021/jo9919052
7.B. C. Ranu, A. Hajra, U. Jana, J. Org. Chem., 2000, 65, 6270-6272. https://doi.org/10.1021/jo000711f
8.J. C. Bussolari, P. A. McDonell, J. Org. Chem., 2000, 65, 6777-677.https://doi.org/10.1021/jo005512a




