Conversion Lithium Metal Fluoride Batteries

Introduction
Conventional lithium -ion batteries (LIB) use intercalation cathode materials, including layered LiCoO2(LCO), spinel LiMn2O4(LMO), olivine LiFePO4 (LFP), layered LiNixMnyCozO2(NMC)[1-2]. However, these intercalation chemistries are rapidly approaching their practically achievable capacities, which is currently the maximum limit on the energy density of most commercially available batteries. LIB is the energy storage device of choice for portable electronics, electric vehicles, and grid-level energy storage, but higher specific power/energy density, longer cycle life, and lower cost are still required [3-6]. Li metal anodes combined with conversion-type lithium cathode chemistries, such as lithium metal fluoride (Li-MF ), due to their higher theoretical potential ( 3.55 vs. Li/Li+ for CuF2 ) and higher gravimetric and volumetric capacities ( FeF3 is 713mAhg -1 and 2196mAhcm -3 ), which has shown great potential to meet such requirements In addition to CuF2 and FeF3 , other metal fluorides, such as FeF2 , CoF2 and NiF2 , have shown higher Theoretical discharge potential and higher volumetric capacity, which are achieved by multiple reversible redox electrochemical conversion reactions enabled by multiple electron transfers per transition metal, as shown in Equation 1 below [8] . In addition, whether it is fluorine or metal elements such as iron and copper, they are all naturally abundant.

Here, "M" represents a transition metal ion, "X" represents a fluoride, and "M" and "n" represent lithium consumption. Despite attractive properties, multiple limitations hinder the practical application of MF cathodes. These obstacles include low electronic conductivity, undesired side reactions with electrolytes, and volume changes during charge and discharge. These features lead to poor capacity reversibility or low Coulombic efficiency, large voltage hysteresis, and rapid capacity degradation during cycling. To cope with these effects, the researchers made extensive adjustments to the MF cathode according to three principles (1) the structural design of the cathode, (2) the stability of the Li metal anode, and (3) the selection of battery components and test conditions.
This article explores the most important concepts in designing electrochemically stable microstructures with wide Li-ion insertion channels and desirable nano morphologies. In addition, the development of more suitable electrolytes is evaluated by optimizing concentration, adding additives and fillers, engineering high-modulus solid electrolytes and polymer electrolytes, and translocation formation of artificial solid electrolyte/electrode interface (ASEI) to stabilize Li metal anodes. In addition, the optimization and modification of battery components, as well as the use of suitable parameters and conditions in battery testing, can provide complementary avenues for improving the performance of MF batteries.
Structural Design of Positive Electrode
Precise control of crystallization, which can effectively prevent particle growth and agglomeration, and thus establish an ideal cathode material nanomorphology (including wide Li-ion insertion channels), all contribute to improving the transport and reaction kinetics of MF cathodes. However, scientists still face challenges in improving electronic conductivity, as traditional architectures based on composite materials or encapsulating nanoscale MFs in three-dimensional (3D) carbon networks still face challenges such as sluggish reaction kinetics and suppressed side reactions. Both binary MFs like CoF2 and ternary MFs like NiyFe1-yF2 show great promise. Likewise, FeF3 or FeF2 exhibited high reversibility and low cost, while CuF2 exhibited a high theoretical potential energy of 3.55 V and a high gravitational energy density of 1874 Wh/kg.10. However, the working potential of FeF2 is low (2.60 V), and CuF2 is irreversible during charge-discharge cycles. The irreversibility of CuF2 is due to the high diffusivity of Cu ions, which leads to phase separation of nanometallic Cu during discharge, and the loss of active species due to Cu ion transport during charge.
On the cathode side, considerable efforts have been made to alleviate the challenges associated with active metal dissolution, electrolyte degradation, electrode volume limitation, and selective fluoride ion permeation. Wu et al. reported a FeF3@C composite with a 3D structure consisting of honeycomb and 3D hexagonal channels. The scanning electron microscope (STEM) and high-resolution transmission electron microscope (HRTEM) of the three-dimensional honeycomb FeF3@C composite are shown in Fig. 1A [9]. The 3D porous framework of carbon and the hexagonal channels within the structure simultaneously achieve rapid electron transfer and lithium-ion transport, respectively, and the size of the honeycomb channels ranges from hundreds of nanometers to several micrometers. The walls of these honeycomb channels are embedded with isolated FeF3 nanoparticles ranging in size from 10-50 nm.
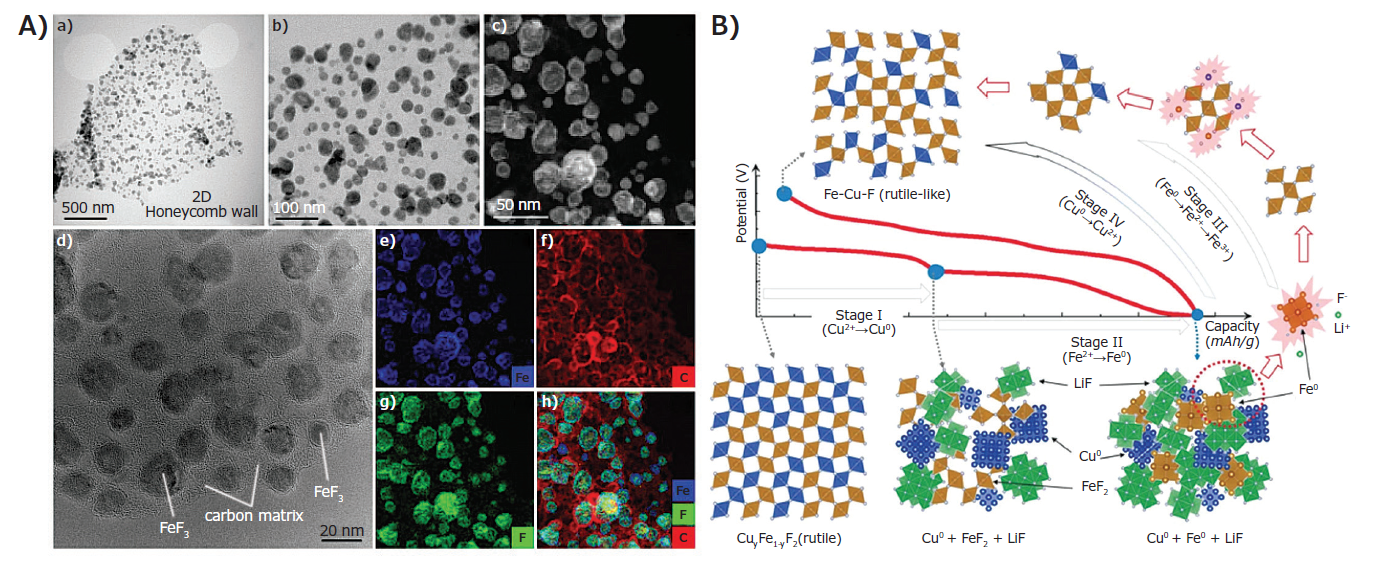
Figure 1 3D STEM and HRTEM images of honeycomb carbon and FeF composites: (a,b) STEM, (c) HRTEM, (d) high-resolution STEM and (eh) corresponding iron (Fe), carbon ( c), Fluorine (F) element and its combinatorial mapping from (d)[9]; B) The reaction pathway of ternary CuyFe1-yF2. The reduction of Cu and Fe at the initial discharge follows phases I and II, while the oxidation of Fe and Cu follows phases III and IV [7].
As a result, this FeF3 @C cathode exhibits a small voltage hysteresis of ~0.30 V at 1C with the following characteristics: a mass loading of 5.3 mgcm-2, 0.25-0.28V, excellent rate capability at 100C, almost no capacity fading at 200 cycles, and ~85% capacity retention after 1000 cycles.
Wang et al. prepared a solid solution ternary MF by substituting Cu into the Fe lattice, and found here a surprisingly small overpotential (<150mV), the addition of Cu promotes a synergistic redox reaction, leading to a reversible reaction Cu2+↔ Cu0. The description of the reaction mechanism and phase evolution is shown in Fig. 1B. This cation substitution provides a new avenue to customize the irreversibility problem in MF cathodes. In stages I and II, Cu and Fe are reduced. In the third stage, transformation of the rutile-like Cu-Fe-F phase occurs. In the higher potential stage (stage IV), most of the Cu converted back to the rutile structure, but a part of Cu was dissolved in the electrolyte or irreversible, implying that there may be a Cu deficiency in the final stage. Later, in order to improve the reversibility of CuF2, Omenya et al. investigated the presence of Fe in Cu1-yFeyF2. The reversibility of Cu0.5Fe0.5F2 is due to the presence of Fe, which exhibits reversible switching and redox reactions in low and high voltage regions, respectively [10].
Stabilization of Lithium Metal Anodes
Researchers in related fields have made considerable efforts to deposit Li metal in a dense and reversible manner, including designing suitable liquid or solid electrolytes, developing stable Li substrate materials, and developing ASEI. The choice of lithium salt, the concentration of the salt, and the composition of the solvent determine the properties of the in situ formed cathode/anode SEI. The design of a suitable liquid electrolyte enhances the structural stability, flexibility, and compatibility between the anode and electrolyte interface. Such an electrolytic solution contains a mixture of LiFSI-DME, acyclic organic carbonate solvent, ADN-FEC solvent, electrolyte additive and nitrile [11-15]. In order to further stabilize the lithium metal anode, it has been reported to use a lithium-ion conductive coating, a solid polymer electrolyte, and a high-fluorine anion-conducting titanite-type La0.9Ba0.1F2.9[1,6,17]. The development of lithium substrate materials and nanostructured scaffolds is another approach to accommodate lithium. Lightweight nanostructures with lithiophilic sites can accommodate enough lithium and promote uniform deposition of lithium, thereby improving cycle performance and reducing voltage response time [5,18]. Besides, the development of ex situ ASEI with high chemical and mechanical stability, high ionic conductivity and Young's modulus, controlling its composition and thickness can lead to dendrite-free Li deposition and stable SEI [3-4]. This leads to the control of Li and liquid electrolyte consumption, suppressing the formation of fragile and unstable, unfavorable, excessively thick SEI. Figure 2 shows a schematic diagram of the SEI formation by drop-casting SnF2 on a Li metal electrode. As the replacement reaction proceeds, SnF2 reacts with Li to form an ASEI composed of Li-Sn alloy, LiF, and electrochemically active Sn that facilitates dendrite-free Li deposition. Moreover, the stable SEI prevents re-precipitation of dissolved metal ions.
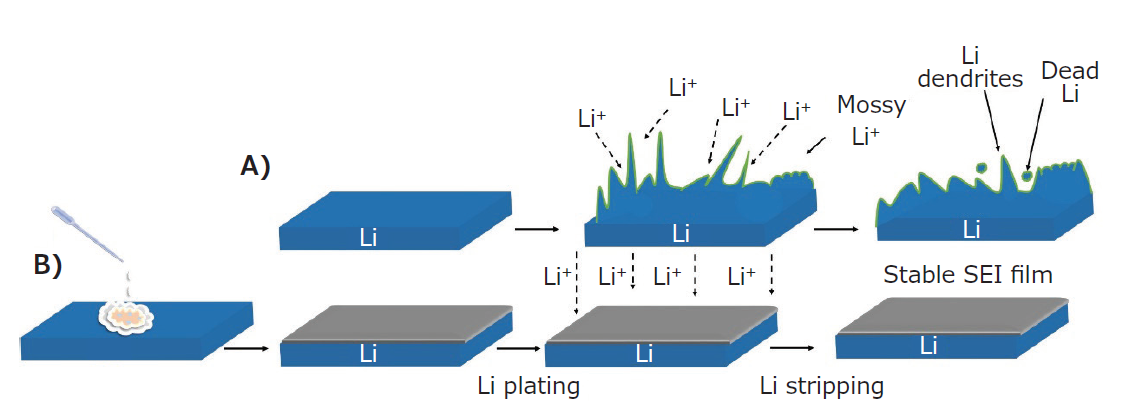
Figure 2 A) Schematic illustration of Li plating/stripping in pure Li and B) SnF2 pretreatment.
Battery Pack Selection and Test Conditions
In addition to the development of advanced 3D cathode structures and Li metal anodes, the design principles of batteries (including the selection of battery components), their modification and testing conditions can also provide complementary avenues to improve the performance of MF batteries. Using suitable carbon additives or binders, the modification of the separator with a lithiophilic coating has the potential to lead to the reversibility of the MF cathode. In addition, battery test conditions, such as determining preferred charge-discharge current densities and charge-discharge voltage limits, can also reduce metal dissolution and/or electrolyte degradation. An increase in temperature during battery testing can prove to be beneficial for the formation of a stable SEI, suppressing Li dendrite growth, and thus enhancing the rate capability and capacity utilization [19].
Conclusions and Future Outlook
Li-MF batteries may be able to meet the rapidly growing demand for lightweight, high-capacity, and high-energy-density storage. Significant research efforts are underway that could lead to safety, low cost, and capacity reversibility, leading to the development of conversion-type Li-MF batteries, which respectively allow storage of nearly twice the volume compared to conventional electrodes energy density and nearly three times the energy density.
Beyond that, future research may continue to focus on fundamental areas of materials discovery and advanced interface characterization. For example, the discovery of novel computational and experimental techniques can help identify lightweight and low-cost cathode structures to further address the problems of poor electronic conductivity, volume expansion, and active metal dissolution. Atomic scale characterization and modeling techniques will help us better understand the desired electrode/electrolyte interface chemistry. Furthermore, the synthesis of cathode structures, in-situ or ex-situ anode/cathode SEI formation, electrolyte optimization, selection of battery components, reproducible protocols and standards for battery fabrication and cycle metrics are critical to the successful deployment of Li-MF battery technology.
Reference
1. Xiao, A. W.; Lee, H. J.; Capone, I.; Robertson, A.; Wi, T.-U.; Fawdon, J.; Wheeler, S.; Lee, H.-W.; Grobert, N.; Pasta, M. Nat. Mater 2020, 19, 644–654. https://www.nature.com/articles/s41563-020-0621-z
2. Wu, F.; Yushin, G. Energy Environ. Sci. 2017, 10 (2), 435–459. https://pubs.rsc.org/en/content/articlelanding/2017/ee/c6ee02326f/unauth
3. Pathak, R.; Chen, K.; Gurung, A.; Reza, K. M.; Bahrami, B.; Pokharel, J.; Baniya, A.; He, W.; Wu, F.; Zhou, Y. Nat. Commun. 2020, 11 (1),1–10. https://www.nature.com/articles/s41467-020-18319-6
4. Pathak, R.; Chen, K.; Gurung, A.; Reza, K. M.; Bahrami, B.; Wu, F.; Chaudhary, A.; Ghimire, N.; Zhou, B.; Zhang, W. H. Adv. Energy Mater. 2019, 9 (36), 1901486. https://onlinelibrary.wiley.com/doi/abs/10.1002/aenm.201901486?casa_token=DQv60xsZJ_8AAAAA:5DQ6DbvwxLzFlov4Dduasge6hnEUUoDCra_hBfoaXgs398IxYoRKvyOvAQPnzCcIdISLi3YateVtx5Le
5. Chen, K.; Pathak, R.; Gurung, A.; Reza, K.; Ghimire, N.; Pokharel, J.; Baniya, A.; He, W.; Wu, J. J.; Qiao, Q. J. Mater. Chem. A 2020, 8, 1911–1919. https://pubs.rsc.org/en/content/articlelanding/2020/ta/c9ta11237e/unauth
6. Chen, K.; Pathak, R.; Gurung, A.; Adhamash, E. A.; Bahrami, B.; He, Q.; Qiao, H.; Smirnova, A. L.; Wu, J. J.; Qiao, Q. Energy Storage Mater. 2019, 18, 389–396. https://www.sciencedirect.com/science/article/pii/S1388248119301985
7. Wang, F.; Kim, S.-W.; Seo, D.-H.; Kang, K.; Wang, L.; Su, D.; Vajo, J. J.; Wang, J.; Graetz, J. Nat. Commun. 2015, 6 (1), 1–9. https://www.nature.com/articles/ncomms7668
8. Prakash, R.; Mishra, A. K.; Roth, A.; Kübel, C.; Scherer, T.; Ghafari, M.; Hahn, H.; Fichtner, M. J. Mater. Chem. 2010, 20 (10), 1871–1876. https://www.mdpi.com/2311-5629/6/2/21
9. Wu, F.; Srot, V.; Chen, S.; Lorger, S.; van Aken, P. A.; Maier, J.; Yu, Y. Adv. Mater. 2019, 31 (43), 1905146. https://onlinelibrary.wiley.com/doi/10.1002/adma.201905146
10. Omenya, F.; Zagarella, N. J.; Rana, J.; Zhang, H.; Siu, C.; Zhou, H.; Wen, B.; Chernova, N. A.; Piper, L. F.; Zhou, G. ACS Appl. Energy Mater. 2019, 2 (7), 5243–5253. https://pubs.acs.org/doi/abs/10.1021/acsaem.9b00938
11. Fan, X.; Hu, E.; Ji, X.; Zhu, Y.; Han, F.; Hwang, S.; Liu, J.; Bak, S.; Ma, Z.; Gao, T. Nat. Commun. 2018, 9 (1), 1–12. https://www.nature.com/articles/s41467-018-04813-5
12. Mäntymäki, M.; Ritala, M.; Leskelä, M. Coatings 2018, 8 (8), 277. https://www.mdpi.com/2079-6412/8/8/277
13. Gu, W.; Borodin, O.; Zdyrko, B.; Lin, H. T.; Kim, H.; Nitta, N.; Huang, J.; Magasinski, A.; Milicev, Z.; Berdichevsky, G. Adv. Funct. Mater. 2016, 26 (10), 1507–1516. https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.201504848
14. Davis, V. K.; Bates, C. M.; Omichi, K.; Savoie, B. M.; Momčilović, N.; Xu, Q.; Wolf, W. J.; Webb, M. A.; Billings, K. J.; Chou, N. H. Science 2018, 362 (6419), 1144–1148. https://pubs.acs.org/doi/full/10.1021/acsaem.1c03375
15. Gmitter, A. J.; Badway, F.; Rangan, S.; Bartynski, R. A.; Halajko, A.; Pereira, N.; Amatucci, G. G. J. Mater. Chem. 2010, 20 (20), 4149–4161. https://pubs.rsc.org/en/content/articlelanding/2010/jm/b923908a/unauth
16. Huang, Q.; Turcheniuk, K.; Ren, X.; Magasinski, A.; Song, A.-Y.; Xiao, Y.; Kim, D.; Yushin, G. Nat. Mater. 2019, 18 (12), 1343–1349. https://www.nature.com/articles/s41563-019-0472-7
17. Thieu, D. T.; Fawey, M. H.; Bhatia, H.; Diemant, T.; Chakravadhanula, V. S. K.; Behm, R. J.; Kübel, C.; Fichtner, M. Adv. Funct. Mater 2017, 27 (31), 1701051. https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.201701051
18. Liu, J.; Yuan, H.; Cheng, X.-B.; Chen, W.-J.; Titirici, M.-M.; Huang, J.-Q.; Yuan, T.; Zhang, Q. Mater. Today Nano 2019, 8, 100049. https://www.sciencedirect.com/science/article/pii/S258884201930118X
19. Yan, K.; Wang, J.; Zhao, S.; Zhou, D.; Sun, B.; Cui, Y.; Wang, G. Angew. Chem. 2019, 131 (33), 11486–11490. https://onlinelibrary.wiley.com/doi/abs/10.1002/ange.201905251
