Friedel–Crafts Acylation
Introduction

Friedel–Crafts Acylation reactions are a class of reactions in which aromatic hydrocarbons are acylated with chlorides or anhydrides under the conditions of Lewis acids as catalysts. This reaction generally does not produce multiple acylation products like alkylation reactions due to the electron-withdrawing effect of the carbonyl group, and only forms single acylation products through electrophilic aromatic substitution. 1,2

Fig. 1. Friedel-Crafts acylation reaction
MECHANISM
Ⅰ. Lewis acid catalyst (AlCl3) forms a complex with the chloride atom of acyl group, and the dissociation of chloride forms acyl carbocation.
Ⅱ. The acylium ion (RCO+) continues to carry out electrophilic attack on aromatic ring. With the formation of the complex, its aromaticity temporarily disappears.
Ⅲ. Deprotonation of intermediate state restores the aromaticity of aromatic ring. Charge is transferred to chloride ion to form HCl, and AlCl3 catalyst is re-formed. 1

Fig. 2. Mechanism of AlCl3 Friedel-Crafts acylation reaction
APPLICATIONS
The Friedel-Crafts acylation reaction can be applied to the synthesis of the following compounds:
1. Diarylacetic acid derivatives 4
2. Polyether ether ketone (PEEK) or mPEK 5
3.1,5-Bis (4-fluorobenzoyl) - 2,6-dimethylnaphthalene 6
4. Aromatic ketone 7
5. Dissymmetric aromatic amines 8
6. Cyclic ketones, such as 1-tetraenone and 1-ninhydrin 9
7.2-acetyl-6-methoxynaphthalene, a key intermediate for the synthesis of new non-steroidal anti-inflammatory and analgesic drugs naproxen and naproxone 10
RESEARCH AND TRENDS
1. PVP-TfOH has been applied as a solid superacid catalyst system with high efficiency and easy post-treatment in Friedel-Crafts acylation reaction. Under mild reaction conditions, aromatic hydrocarbons and glyoxylic acid were synthesized by a solvent-free one-pot method through Friedel-Crafts acylation reaction. 4
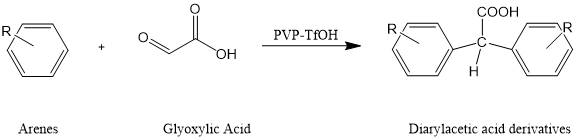
Figure 3. Diarylacetic acid derivatives are synthesized by solvent-free one-pot method through Friedel-Crafts acylation reaction of aromatic hydrocarbons and glyoxylic acid
2. An imidazole-based ionic liquid can be used as a catalyst to catalyze the Friedel-Crafts acylation of aromatic hydrocarbons with acetyl chloride .11
3. It is reported that erbium trifluoromethanesulfonate is a highly efficient catalyst containing electron donor groups and can be used for microwave assisted Friedel-Crafts acylation of aromatics. 12
4. Phoebe tigrina alkaloids are a kind of highly complex and diverse triterpenoid alkaloids, which can directly and rapidly construct its ACDE ring system through intramolecular Friedel-Crafts acylation reaction . 13
5. The acid-catalyzed domino Friedel-Crafts acylation reaction can be used to efficiently construct the 6,5,6-ABC tricyclic skeleton structure of Taiwan Taxoquinone. It can also be used for the synthesis of diterpenoids (±) - menadione B and (±) - dichlorone. 14
6. Two monomers containing 1,4-naphthalene units, new poly (aryl ketone) and poly (aryl ether ketone sulfone), can be synthesized by electrophilic Friedel-Crafts acylation polycondensation. 15
7. According to research, the toluene and acetic anhydride of triphenyltin in SBA-15 can undergo Friedel-Crafts acylation reaction. 16
8.The indium trifluoromethanesulfonate in the ionic liquid 1-isobutyl-3-methylimidazolium dihydrophosphate ([i-BMIM] H2PO4) shows strong catalytic activity in the Friedel-Crafts acylation reaction of aromatic compounds with anhydride. 17
9.Friedel-Crafts acylation of 25,27-dialkyloxycalixarene. Acyl chloride and AlCl3 are used to directly acylate the tertiary butylcalixarene in 1,2-dichloroethane to provide corresponding diacyl derivatives with high yield and regioselectivity. 18
References
1.Fox MA, Whitesell JK. 1994. Organic Chemistry. Boston: Jones and Bartlett.
2.Li JJ. 2009. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. Springer.
3.Sartori G, Maggi R. Advances in Friedel-Crafts Acylation Reactions. https://doi.org/10.1201/9781420067934
4.Prakash G, Paknia F, Kulkarni A, Narayanan A, Wang F, Rasul G, Mathew T, Olah GA. 2015. Taming of superacids: PVP-triflic acid as an effective solid triflic acid equivalent for Friedel?Crafts hydroxyalkylation and acylation. Journal of Fluorine Chemistry. 171102-112. https://doi.org/10.1016/j.jfluchem.2014.08.020
5.Baek J, Lyons CB, Tan L. 2004. Grafting of Vapor-Grown Carbon Nanofibers via in-Situ Polycondensation of 3-Phenoxybenzoic Acid in Poly(phosphoric acid). Macromolecules. 37(22):8278-8285. https://doi.org/10.1021/ma048964o
6.Ohno M, Takata T, Endo T. 1995. Synthesis of a novel naphthalene-based poly(arylene ether-ketone) by polycondensation of 1,5-bis(4-fluorobenzoyl)-2,6-dimethylnaphthalene with bisphenol a. J. Polym.Sci. A Polym.Chem.. 33(15):2647-2655. https://doi.org/10.1002/pola.1995.080331511
7.de Noronha RG, Fernandes AC, Romão CC. 2009. MoO2Cl2 as a novel catalyst for Friedel?Crafts acylation and sulfonylation. Tetrahedron Letters. 50(13):1407-1410. https://doi.org/10.1016/j.tetlet.2009.01.039
8.Nordlander JE, Payne MJ, Njoroge FG, Balk MA, Laikos GD, Vishwanath VM. 1984.Friedel-Crafts acylation with N-(trifluoroacetyl)-.alpha.-amino acid chlorides.Application to the preparation of .beta.-arylalkylamines and 3-substituted 1,2,3,4-tetrahydroisoquinolines. J.Org.Chem.. 49(22):4107-4111. https://doi.org/10.1021/jo00196a001
9.Tran PH, Huynh VH, Hansen PE, Chau DN, Le TN. 2015. An Efficient and Green Synthesis of 1-Indanone and 1-Tetralone via Intramolecular Friedel-Crafts Acylation Reaction. Asian Journal of Organic Chemistry. 4(5):482-486. https://doi.org/10.1002/ajoc.201402274
10.Kobayashi S, Komoto I. 2000. Remarkable Effect of Lithium Salts in Friedel?Crafts Acylation of 2-Methoxynaphthalene Catalyzed by Metal Triflates. Tetrahedron. 56(35):6463-6465. https://doi.org/10.1016/s0040-4020(00)00610-4
11.Cai M, Wang X. 2014. Activity of Imidazolium-Based Ionic Liquids as Catalysts for Friedel-Crafts Acylation of Aromatic Compounds. Asian J. Chem.. 26(18):5981-5984. https://doi.org/10.14233/ajchem.2014.16354
12.Tran PH, Hansen PE, Nguyen HT, Le TN. 2015. Erbium trifluoromethanesulfonate catalyzed Friedel?Crafts acylation using aromatic carboxylic acids as acylating agents under monomode-microwave irradiation. Tetrahedron Letters. 56(4):612-618. https://doi.org/10.1016/j.tetlet.2014.12.038
13.Wang W, Li G, Wang S, Shi Z, Cao X. 2015. Direct and Short Construction of the ACDE Ring System of Daphenylline. Chem. Asian J.. 10(2):377-382. https://doi.org/10.1002/asia.201403152
14.Tang S, Xu Y, He J, He Y, Zheng J, Pan X, She X. 2008. Application of a Domino Friedel?Crafts Acylation/Alkylation Reaction to the Formal Syntheses of (±)-Taiwaniaquinol B and (±)-Dichroanone. Org.Lett.. 10(9):1855-1858. https://doi.org/10.1021/ol800513v
15.Wen H, Wang P, Cheng S, Yan T, Cai M. 2015. Synthesis and characterization of novel organosoluble poly(aryl ether ketone)s and poly(aryl ether ketone sulfone)s containing 1,4-naphthylene units. High Performance Polymers. 27(6):705-713. https://doi.org/10.1177/0954008314557707
16.Deng Q, Qin Z, Yang Y, Song W. 2015. Synthesis, characterization of triphenyltin grafted on SBA-15 mesoporous silica and its catalytic performance for the synthesis of 4-methylacetophenone. Chinese Journal of Chemical Engineering. 23(2):384-388. https://doi.org/10.1016/j.cjche.2013.12.001
17.Tran PH, Hansen PE, Hoang HM, Chau DN, Le TN. 2015. Indium triflate in 1-isobutyl-3-methylimidazolium dihydrogen phosphate: an efficient and green catalytic system for Friedel?Crafts acylation. Tetrahedron Letters. 56(17):2187-2192. https://doi.org/10.1016/j.tetlet.2015.03.051
18.Skácel J, Budka J, Eigner V, Lhoták P. 2015. Regioselective Friedel?Crafts acylation of calix[4]arenes. Tetrahedron. 71(13):1959-1965. https://doi.org/10.1016/j.tet.2015.02.021
Aladdin:https://www.aladdinsci.com
List of related products
