JAK-STAT Cell Signaling Pathway
The Janus kinase (JAK) signal transduction and transcriptional activator (STAT) pathway plays a key role in the signaling of a large number of cytokines and growth factors. These cytokines and growth factors are responsible for a variety of cellular functions, including proliferation, growth, hematopoietic and immune responses [1-4]. Thermo Scientific™ has a wide range of products to help with JAK-STAT research. Binding of cytokines and growth factors to corresponding receptors activates JAK, which then phosphorylates STAT proteins on the receptor and specific tyrosine residues. Then STATs dimerization, translocation to the nucleus, binding to the consistent DNA 5 '-TT(N4-6)AA-3' sequence, initiating the transcription of target genes [1-4]].
Key JAK-STAT Pathway Targets
Four JAK family kinases and seven STAT family members have been identified:
JAK1 | STAT4 |
JAK2 | STAT5a |
JAK3 | STAT5b |
STAT1 | STAT6 |
STAT2 | TYK2 |
STAT3 |
Common targets in the JAK/STAT Pathway:
EGF | IL-6 |
GM-CSF | IL-12 |
GRB2 | IL-13 |
IFN-α/β | PIAS |
IFN-γ | Shc |
IL-2 | SHP |
IL-3 | SOCS3 |
IL-4 |
JAK1, JAK2, and TYK2 appear to be universally expressed, while JAK3 expression is usually limited to lymphocytes. JAKs is structurally unique in that it is preceded by a pseudo-kinase domain (JH2) at the C-terminal kinase domain (JH1) that lacks catalytic activity but has key regulatory functions. JAKs also has the Src homology 2 (SH2) domain and the N-terminal 4:1, ezrin, radixin, moesin (FERM) domain, which is critical for mediating cytokine receptor association.
The STAT protein contains a SH2 domain for dimerization and a DNA-binding domain. The diversity of amino acid sequences and their tissue-specific distribution explain the different effects of STATs in response to extracellular cytokines. [1-4] The JAK-STAT pathway is upregulated by a large number of cytokines/growth factors. One mechanism by which the JAK-STAT pathway is negatively regulated is through cytokine signaling inhibitor proteins (SOCS), which directly bind and inactivate JAKs[5], and activated STATs protein inhibitors (PIAS), which bind to phosphorylated STAT dimers and prevent DNA from binding [6].
Abnormal constitutive activation of the JAK-STAT pathway has been associated with various cancers and immune disorders. For example, STAT3 and STAT5 are overactive in many tumors, including major tumors and some hematologic tumors[7,8].Activation of the JAK2 mutation is associated with leukemia. TEL-JAK2 fusion resulting from chromosomal translocation was found in a small group of human T-cell acute lymphoblastic leukemia patients[9].The V617F mutation in the JH2 pseudokinase domain of JAK2 has been found in a high proportion of patients with myeloproliferative diseases, including polycythemia vera[10]. Inhibitors of the JAK-STAT pathway are currently under development in the fields of oncology and immune disorders.
FIGURES
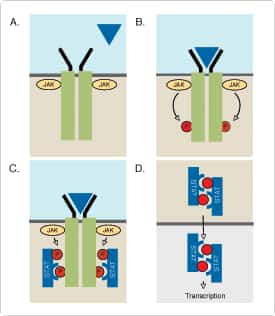
Events that cause STAT activation. (A) Extracellular interferon before binding to the receptor (blue triangle). (B) After binding, JAK kinases associated with the composition of the receptor subunit interact. Interacting JAK proteins activate each other by mutual tyrosine phosphorylation and phosphorylate tyrosine on two subunits contained in the receptor complex. These phosphorylated tyrosine residues provide a paired docking site (C) for STAT through their SH2 domain. STAT is recruited to the receptor complex and then phosphorylated by JAKs on tyrosine residues. This tyrosine phosphorylation promotes the formation of STAT homologous and heterodimers through phosphorylated tyrosine SH2 domain interactions and dimer separation from the receptor complex (D). The STAT dimer is then transferred to the nucleus, where it combines with other nucleoproteins and regulates gene expression by binding to promoters or other responsive elements on the DNA.
References
1. Aaronson, D.S., et al. (2002) A road map for those who don’t know JAK-STAT. Science 296: 1653-1655.
2. Rybinski, M. (2012) Model-based selection of the robust JAK-STAT activation mechanism. J Theoretical Bio 309: 34-46.
3. O’Shea, J.J., et al. (2004) A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discovery 3: 555-564.
4. Liongue, C., et al. (2012) Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS ONE 7: e32777.
5. Kishimoto, T., et al. Knocking the SOCS off a tumor suppressor. (2001) Nature Genetics 28: 4-5.
6. Shuai, K,. et al. (2000) Modulation of STAT signaling by STAT-interacting proteins. Oncogene 19 : 2638-2645.
7. Darnell, J.E., et al. (2005) Validating stat3 in cancer therapy. Nature Medicine 11: 595-596.
8. Yu, H., et al. (2004) The STATs of cancer – new molecular targets come of age. Nat Rev Cancer 4: 97-105.
9. Lacronique, V., et al. (1997) A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278: 1309-1312.
10. Ferrajoli, A., et al. (2006) The JAK-STAT pathway: a therapeutic target in hematological malignancies. Current Cancer Drug Targets 6: 671-679.
Aladdin:https://www.aladdinsci.com
List of related products
