Knoevenagel Condensation Reaction

Organic chemistry is a vast field that encompasses various synthetic pathways for producing complex molecules. One method is the Knoevenagel condensation reaction. This is a versatile reaction involving the condensation of carbonyl compounds and active methylene compounds to form α,β-Unsaturated carbonyl compounds.
Knoevenagel Condensation Reaction
Knoevenagel condensation reaction is a classic organic named reaction involving the reaction of aldehydes or ketones with active methylene compounds such as malonates or ethyl acetoacetate in the presence of alkaline catalysts. This reaction forms α,β-Unsaturated carbonyl compounds.1 This reaction was first described by German chemist Emil Knoevenagel in 1894, hence its name.

Figure 1. Knoevenagel Condensation Reaction
Z, Z' (electron withdrawing groups) = CO2R, COR, CHO, CN, NO2, etc.
Mechanism of the Knoevenagel Condensation
The mechanism of Knoevenagel condensation can be divided into the following three steps:
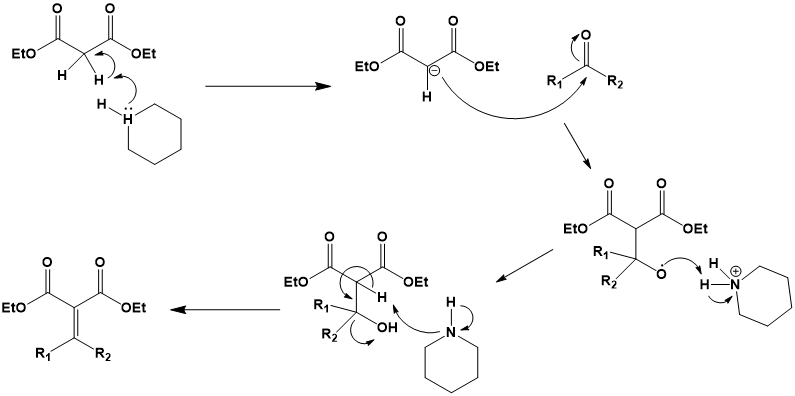
Figure 2. Mechanism of the Knoevenagel Condensation
Step 1: The first step is to use basic catalyst to α-Carbon undergoes deprotonation to form carbon negative ions. The base catalyst abstracts the acidic proton from the α-carbon produces negatively charged carbon negative ions.
Step 2: Nucleophilic attack In the second step, carbon negative ions attack the carbonyl groups of aldehydes or ketones, forming tetrahedral intermediates. Negatively charged carbon negative ions act as nucleophilic reagents, attacking the electrophilic carbon of carbonyls, promoting the formation of intermediates, which contain new carbon carbon bonds.
Step 3: Elimination In the last step, the tetrahedral intermediate undergoes a elimination reaction to remove the leaving group (usually alcohol molecule) and form α,β- Unsaturated carbonyl compounds. The elimination reaction involves the breaking of the carbon oxygen bond of the leaving group, and the leaving group leaves in the form of an alcohol molecule. The final product contains carbon carbon double bonds conjugated with carbonyl groups α,β- Unsaturated carbonyl compounds.
Factors Affecting Knoevenagel Condensation
The following important factors can affect the Knoevenagel reaction:
1. Concentration of reactants: the concentration of carbonyl compounds and active methylene compounds will affect the reaction rate. The higher the concentration of reactants, the faster the reaction rate.
2. Alkaline catalyst type: The type and concentration of alkaline catalyst used in the reaction will affect the reaction rate and selectivity. Strong bases such as sodium hydride (NaH) and potassium tert butanol (t-BuOK) are typically used in Knoevenagel condensation reactions.
3. Solvent: The choice of solvent also affects the reaction rate and selectivity. Polar non proton solvents such as dimethyl sulfoxide (DMSO) and N, N-dimethylformamide (DMF) play an important role in promoting the reaction.
4. Temperature: The reaction rate of Knoevenagel condensation reaction is dependent on temperature. Higher temperatures usually lead to faster reaction rates, but also increase the likelihood of side reactions and unwanted by-products.
5. Water content of reactants: water will affect Knoevenagel condensation reaction by hydrolyzing carbonyl compounds and/or active methylene compounds. Therefore, it is important to ensure that the reaction mixture is anhydrous.
By controlling these factors, the Knoevenagel reaction can be optimized to produce the required products with high selectivity and yield.
Applications of Knoevenagel Condensation
The Knoevenagel condensation reaction uses primary and secondary amines and their salts as catalysts, laying an early foundation for the study of amino catalysts.2 The research on new catalysts and activation methods for Knoevenagel condensation reaction is still ongoing:
Microwave and ultrasonic irradiation reactions
The improvement of the Knoevenagel condensation reaction of 3-isochromone and aromatic aldehydes can be achieved by changing the ratio of E/Z isomers by microwave radiation on a solid carrier in the presence of various catalysts.3
Solvent free conditions
In the absence of solvent, arylene methylenes can be synthesized rapidly and efficiently by the Knoevenagel condensation reaction using aldehydes and reactive methylene compounds as reaction materials, catalyzed by catalytic amounts of lithium hydroxide (LiOH-H2O).4
Photochemical condensation using fruit extracts as catalysts
With aqueous solutions of tamarind juice as natural catalysts, visible light can induce both efficient and environmentally friendly Knoevenagel condensation reactions of various aliphatic and aromatic aldehydes with malononitrile with high reaction yields.5
Under visible light, aqueous starfruit juice solution can catalyze the Knoevenagel condensation reaction of aromatic aldehydes with malononitrile equally simply and effectively. 6
Limitations of Knoevenagel Condensation
Although the Knoevenagel condensation reaction is a practical reaction with many areas of application, there are some limitations:
1. Limited reaction range: The Knoevenagel reaction is mainly limited to the reaction of aldehydes or ketones with reactive methylene compounds. Other types of carbonyl compounds, such as esters or amides, are usually not reactive under the reaction conditions.
2. By-product formation: Knoevenagel reactions can produce unwanted by-products such as Michael addition products, hydroxyl aldol condensation products and polymerization products. These by-products can reduce the yield and purity of the target product.
3. Reaction sensitivity: The Knoevenagel reaction is sensitive to reaction conditions, such as temperature, concentration and reaction time. Small changes in these parameters can lead to significant changes in the reaction results.
4. Stereoselectivity: The Knoevenagel reaction is usually not stereoselective, which means that it can produce a mixture of stereoisomers. This can be a limitation when stereoselective synthesis is required.
5. Product stability: Some products of Knoevenagel reactions may be unstable and prone to decomposition or rearrangement under certain conditions. This may limit their applicability in certain areas, such as in pharmaceutical applications.
Despite these limitations, Knoevenagel reactions are still a valuable tool in organic synthesis, especially for the synthesis of α,β-unsaturated carbonyl compounds. By understanding the limitations of the reaction, the reaction conditions can be further optimized to obtain the desired experimental results.
Reference
1.March J. 1968. in Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, McGraw-Hill. 693697-698.
2.List B. 2010. Emil Knoevenagel and the Roots of Aminocatalysis. Angew. Chem. Int. Ed.. 49(10):1730-1734. https://doi.org/10.1002/anie.200906900
3.Vass A, Földesi A, Lóránd T. 2006. Reactions of 3-isochromanone with aromatic aldehydes?microwave assisted condensations performed on solid basic inorganic supports. Journal of Biochemical and Biophysical Methods. 69(1-2):179-187. https://doi.org/10.1016/j.jbbm.2006.03.011
4.Pasha MA, Manjula K. 2011. Lithium hydroxide: A simple and an efficient catalyst for Knoevenagel condensation under solvent-free Grindstone method. Journal of Saudi Chemical Society. 15(3):283-286. https://doi.org/10.1016/j.jscs.2010.10.010
5.Pal R. Visible light induced Knoevenagel condensation: A clean and efficient protocol using aqueous fruit extract of tamarindus indica as catalyst. 2(1): https://doi.org/10.14419/ijac.v2i1.1703
6.Pal R, Sarkar T. 2014. Visible Light Induced Knoevenagel Condensation Catalyzed by Starfruit Juice of <i>Averrhoa carambola</i>. IJOC. 04(02):106-115. https://doi.org/10.4236/ijoc.2014.42012
