N-Heterocyclic Carbene (NHC) Ligands

With rapid advances in the field of cross-coupling reactions of unactivated substrates catalyzed by metal complexes, the chemical market has been revolutionized by the introduction of a large library of achiral and chiral phosphine ligand compounds. Phosphine ligands have shown positive effects in numerous reports of efficient systems for conversion and selectivity in conventional industrial processes such as hydrogenation. However, until now, the drawbacks of the high production cost of tertiary (especially chiral) phosphine and its tendency to degrade into phosphine oxide have been addressed by the rich field of NHC ligands for homogeneous catalysis.1
Advantages of n-heterocyclic carbene as a helper ligand include:
1. They are stronger s-donors than phosphine and are beneficial to palladium-catalyzed oxidation addition rate of aryl chloride;
2. The strong metal-Carbene bond of NHC complex facilitates tight binding kinetics, thus reducing ligand dissociation;
3. The presence of steric groups bonded to the N atom contributes to the reduction elimination of products from palladium;
4. As we have seen in the synthesis of benzimidazolidins containing electron-dissimilar groups on the aromatic backbone, the activity of NHC ligands can be modified by the remote introduction of electron-directed substituents.2
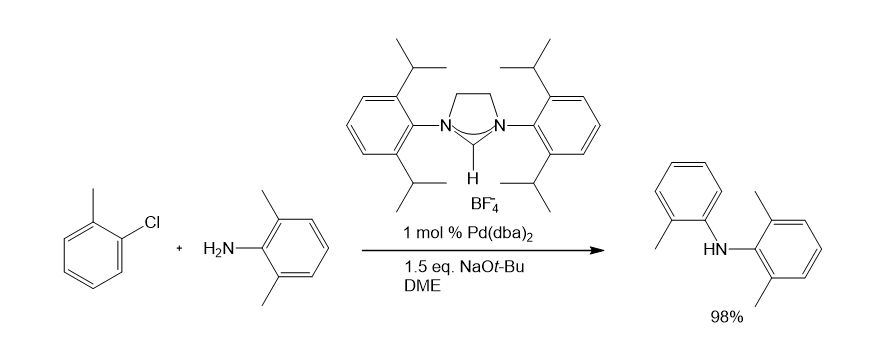
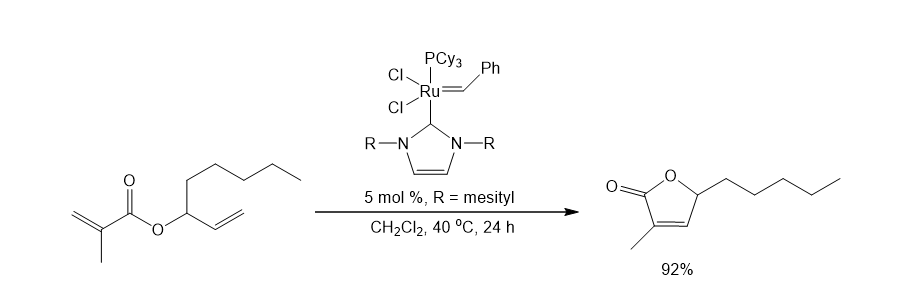
A wide range of NHC ligands that exhibit high activities in various important organic transformations when combined with metal pre-catalysts are now commercially available. NHC imidazolidine ligands with sterically encumbering groups such as mesityl, isopropyl, and adamantyl have been used in the Pd-catalyzed cyclization of anilides,3 amination of aryl chlorides,4 arylation with ester enolates to afford a-aryl esters,5 Sonogashira reactions of unactivated alkyl bromides,6 and the ruthenium-catalyzed RCM reaction (Schemes 1-5).7 The last example shows the ability of NHC ligands to stabilize metal-active substances in solution, thus giving the catalyst the ability to RCM highly substituted and electron-poor olefin reactants to produce tetrad substituted products. Such remarkable reactivity is unique to Shrock alkylene catalysts.8 However, the use of these imidazolidins results in better performance than earlier ruthenium decomposition catalysts and provides higher stability than the Shrock system. The goal of Aladdin® is to accelerate the development of cutting-edge research in NHC ligand-mediated chemistry, and to do so, we offer NHC ligands with a variety of different spatial requirements.
Scheme 2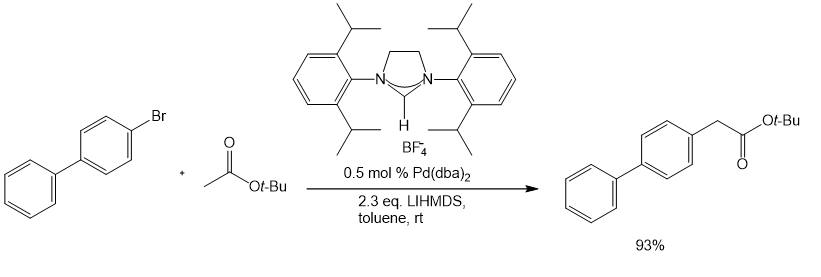
Scheme 3
Scheme 4
Scheme 5
Reference1. Herrmann. 2002. et al. Angew. Chem.. Int. Ed. Engl.. 41, 1290.
2. Hadei N, Kantchev EAB, O'Brie CJ, Organ MG. 2005. Electronic Nature of N-Heterocyclic Carbene Ligands: Effect on the Suzuki Reaction. Org. Lett.. 7(10): 1991-1994. https://doi.org/10.1021/ol050471w
3. Lee S, Hartwig JF. 2001. Improved Catalysts for the Palladium-Catalyzed Synthesis of Oxindoles by Amide α-Arylation.Rate Acceleration, Use of Aryl Chloride Substrates, and a New Carbene Ligand for Asymmetric Transformations. J. Org. Chem.. 66(10): 3402-3415. https://doi.org/10.1021/jo005761z
4. Stauffer SR, Lee S, Stambuli JP, Hauck SI, Hartwig JF. 2000. High Turnover Number and Rapid, Room-Temperature Amination of Chloroarenes Using Saturated Carbene Ligands. Org. Lett.. 2(10):1423-1426. https://doi.org/10.1021/ol005751k
5. Jørgensen M, Lee S, Liu X, Wolkowski JP, Hartwig JF. 2002. Efficient Synthesis of α-Aryl Esters by Room-Temperature Palladium-Catalyzed Coupling of Aryl Halides with Ester Enolates. J. Am. Chem. Soc.. 124(42): 12557-12565. https://doi.org/10.1021/ja027643u
6. Eckhardt M, Fu GC. 2003. The First Applications of Carbene Ligands in Cross-Couplings of Alkyl Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides. J. Am. Chem. Soc.. 125(45): 13642-13643. https://doi.org/10.1021/ja038177r
7. Fürstner A, Thiel OR, Ackermann L, Schanz H, Nolan SP. 2000. Ruthenium Carbene Complexes with N,N’-Bis(mesityl)imidazol-2-ylidene Ligands: RCM Catalysts of Extended Scope. J. Org. Chem.. 65(7):2204-2207. https://doi.org/10.1021/jo9918504
8. Schrock RR, Murdzek JS, Bazan GC, Robbins J, DiMare M, O'Regan M. 1990. Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. J. Am. Chem. Soc.. 112(10): 3875-3886. https://doi.org/10.1021/ja00166a023