N2 Column
Spcifications for N2 Columns
Application : Analysis and purification by HPLC of fluorophore and UV-chromophore labeled glycans.
Description: N2 HPLC columns contain particles with a polymeric amide coating optimized for high resolution chromatography of complex glycan mixtures.
Particles: 3 μm particle size with 80 angstrom pores and polymeric amide coating.
Column Size Cat # Dimensions
C498853-01 2.0 x 150 mm
C498853-02 4.6 x 150 mm
Flow Rates:Typical flow rates = 0.4 - 1.0 ml/min for 4.6mm column 0.15 - 0.22ml/min for 2.0mm column
Column Pressure Maximum pressure = 2250 psi (150 kg/cm2 ) for 4.6mm column 2900 psi (195 kg/cm2 ) for 2.0mm column
pH Range:2.0 - 7.5
Temperature: Typical operating temperature = 30 ℃. Temperature range = 10 - 40℃.
Solvents: Typical solvent systems for glycan analysis include gradients of acetonitrile and aqueous buffers containing ammonium formate, pH 4.4. The recommended buffer is 50 mM ammonium formate pH 4.4 which is available in 50 ml aliquots for making up 2 liters.(阿拉丁 货号#:N498870-50ml)
Shipping Solvent : 75% acetonitrile - 25% water
Storage:For long-term storage, the column should be washed with a gradient going from the operational buffer to 100% water then to the storage solvent of >50% acetonitrile in water (v/v).
Column Protection: Filter all solvents to 0.2 μm and degas using either helium sparging or vacuum degassing. Filter all samples using a 0.2 μm filter membrane before loading onto the column. Install an in-line filter between the sample injector and the column.
Amount of Sample :The maximum amount of glycan sample that can be loaded on the column depends on the number and type of glycan components as well as the nature of any non-glycan material. The typical range for successful analytical runs is 10fmol - 1 nmol per sample peak and up to 200 nmol of total glycans.
Suitable Samples: Suitable samples include glycans labeled with the following labels : 2-AA (2-aminobenzoic acid), 2-AB (2-aminobenzamide), AA-Ac (3-(acetylamino)-6- aminoacridine
Sample: Filter samples to 0.2 μm then dry using a centrifugal evaporator.
Preparation:For the 4.6mm column inject the sample in up to 100 μl of the starting buffer (i.e. the solvent mixture used at the very start of the HPLC gradient). For the 2mm column inject the sample in up to 25 μl of the starting buffer (i.e. the solvent mixture used at the very start of the HPLC gradient) .
Sample Detection: Either fluorescence, mass spectrometry or UV-absorbance depending on the dye used.
HPLC System Requirements
N2 columns can be used with an HPLC system capable of delivering accurate gradients at a flow rate of 0.3 to 1.0 ml/min for the 4.6 mm diameter column, or 50 to 300 μl/min for the 2mm diameter column. In general, systems which mix eluants at high pressure (after the pump head) have lower dead volumes and supply more accurate gradients that are appropriate at the flow rate needed for columns.
For the 4.6mm column inject the sample in up to 100 μl of the starting buffer (i.e. the solvent mixture used at the very start of the HPLC gradient). For the 2mm column inject the sample in up to 25 μl of the starting buffer.
A fluorescence detector is required with the following detection wavelengths:
Fluorescence Label | λex (nm) | λex (nm) |
2-AB[2-aminobenzamide] | 330 | 420 |
2-AA [2-aminobenzoic acid] | 330 | 420 |
AA-Ac [3-(acetylamino)-6-aminoacridine] | 442 | 525 |
For optimal detection, use wide slit widths (e.g. 10 – 20 nm). Sub-picomole levels of 2-AB or 2-AA labelled glycans can be detected with good signal-to-noise (depending on the sensitivity of the detector used).
To improve repeatability and intermediate precisions for glycan analyses use a column temperature controller. Good results can be obtained with a column temperature of 35℃.
Installation of the Column
During column installation we recommend that :
• You should connect the N2 column to your HPLC system using standard 1/16” OD tubing and 10-32 (1/16”) fittings in either stainless steel or PEEK (polyetheretherketone). Finger-tight PEEK fittings and tubing (0.17 mm / 0.007” ID) are recommended for ease of connection and to minimise damage to the column threads. Flow direction is indicated by an arrow on the column label.
• Keep the lengths of tubing between the injector to column and column to detector as short as possible to minimise dispersion effects.
• Install an in-line 0.2 μm filter with minimal dead volume between the injector and the head of the N2 column to prevent damage to the column by particles.
• Where two 150 x 2mm columns are joined together use as short a length of tubing as possible.
• Before analysing any samples, condition your newly installed column as follows.
Column Cleaning and Storage
After heavy use, you N2 column may become contaminated with strongly adsorbed sample constituents that will lead to a loss in column performance.
Sample Preparation
N2 columns should be used for analysis of purified glycans (fluorescently labeled or unlabeled).
Samples must be free of particulates. The sample should be dissolved in water before addition of acetonitrile to make up to the same composition as the start of the gradient (e.g. dissolve sample in 35 μl water, then add 65 μl acetonitrile before injection to run with the N-glycan analysis gradient method.
Fluorescent Labeling
Fluorescent glycans can be prepared by derivatizing pure glycans using reductive amination with a label and purification on a cartridge.
The following are commonly used labeling and purification systems for biopharmaceutical glycosylation analysis.
Labeling System | Post-Labeling Purification System |
2-AB | S-Cartridge |
2-AA | S-Cartridge |
AA-Ac | D1-Cartridge |
Filtering Samples
Remove particulates from samples by filtering through a spin filter or syringe filter with 0.2 μm pore size membrane.
Sialylated Glycans
Sialylated glycans can become desialylated if exposed to acidic conditions and elevated temperatures. Avoid desialylation with such samples by
a. minimizing exposure to acid (if possible, keep the pH between 5 - 8), and
b. minimizing exposure to temperatures greater than 25℃.
Operating Schedule and System Suitability Test for 4.6 x 150 mm Column
• Two running methods are listed below: the 30 min run starting at 35% A is suitable for analysis of N-glycans; the 60 min run starting at 20% A is suitable for analysis of both O-glycans and N-glycans
• When starting the analysis bring the flow rate up slowly by running the startup method: N2-10m-Start-35% for N-glycan analysis or N2-10m-Start-20% for O-glycan and N-glycan analysis
• Follow this by running a complete gradient with no sample to condition the column with the running method: N2-30m-Nlink-35% : N-Glycan Analysis Gradient or N2-60m-ONlink-20% (see below for gradient methods)
• Run one of the following system suitability standards using the N2-30m-Nlink-35% or N2-60m-ONlink-20% gradient: 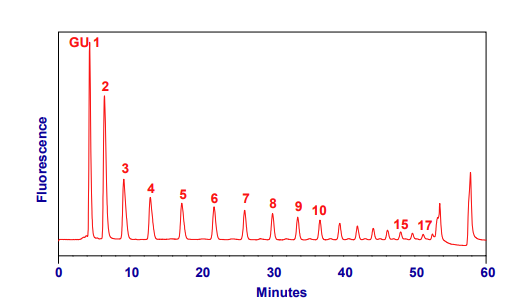
Figure 1: Typical HPLC chromatogram from 2-AB GHP run with method N2-30m-Nlink-35% on a 4.6 x 150 mm N2 column.
Sample injected in 35%aqueous, 65% acetonitrile.
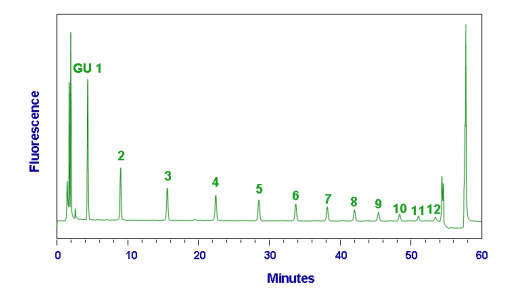
Figure 2: Typical HPLC chromatogram from 2-AB GHP run with method N2-60m-Nlink-20% on a 4.6 x 150 mm N2 column.
Sample injected in 20%aqueous, 80% acetonitrile.
• The GHP system suitability profile should be similar to that in figure 1 using the gradient N2-30m-Nlink-35%, or figure 2 if using the gradient N2-60m-Nlink-20%,.
• If all 12 peaks do not elute in this time, then this is likely to be due to a high dead volume in the HPLC system. Increase the run time to take this into account. (e.g. gradient of 35-47% A over 24 min).
• The peaks should be symmetrical, if there is tailing or the peaks are too broad then try cleaning the column (see Section: Column Cleaning and Storage) or change to a new column.
• Continue running fluorescent labelled GHP suitability standards approximately every 10 sample runs.
• The GHP ladder can also be used to calibrate against day-to-day and system-to-system changes by expressing the elution time of peaks as glucose units (GU). The GU value is calculated by fitting a cubic spline distribution curve to the GHP ladder (GU 1-12 in figure 1), this curve is then used to allocate GU values from retention times. These GU values are very reproducible for neutral glycans (+/- 0.03), with more variation in sialylated glycans (+/- 0.3) and can be compared to database values.
Glycan Analysis with the 4.6 x 150 mm LS-N2 Amide HPLC Column
Solvents
The glycan analysis gradients in this guide are based on the following solvents:
Solvent A : 50 mM ammonium formate pH 4.4, (50 ml of N-BUFFX40 diluted to 2 litres with water)
Solvent B : Acetonitrile
Gradient N2-10m-Start-35% : N-Glycan Analysis Startup
Use as a start up method for gradient for analysis of N-glycans which starts at 35% A.
Time (min) | % A | %B | 4.6mm column Flow Rate (ml/min) |
0 | 35 | 65 | 1.0 |
2 | 35 | 65 | 1.0 |
10 | 35 | 65 | 1.0 |
Gradient N2-30m-Nlink-35% : N-Glycan Analysis Gradient
Use as a gradient for analysis of N-glycans with GU between 4 and 12
Time (min) | % A | %B | 4.6mm column Flow Rate (ml/min) |
0 | 35 | 65 | 1.0 |
22 | 46 | 54 | 1.0 |
22.5 | 100 | 0 | 1.0 |
24.5 | 100 | 0 | 1.0 |
26 | 35 | 65 | 1.0 |
30 | 35 | 65 | 1.0 |
Gradient N2-10m-Start-20% : O-Glycan and N-Glycan Analysis Startup
Use as a start up method for gradient for analysis of O-glycans and N-glycans which starts at 20% A.
Time (min) | % A | %B | 4.6mm column Flow Rate (ml/min) |
0 | 20 | 80 | 0.0 |
2 | 20 | 80 | 1.0 |
10 | 20 | 80 | 1.0 |
Gradient LS-N2-60m-ONlink-20% : O-Glycan and N-Glycan Analysis Gradient
Use as a gradient for analysis of O-glycans and N-glycans with GU between 1 and 12.
Time (min) | % A | %B | 4.6mm column Flow Rate (ml/min) |
0 | 20 | 80 | 1.0 |
52 | 46 | 54 | 1.0 |
52.5 | 100 | 0 | 1.0 |
54.5 | 100 | 0 | 1.0 |
54 | 20 | 80 | 1.0 |
60 | 20 | 80 | 1.0 |
Operating Schedule and System Suitability Test for two 2.0 x 150 mm Columns
Two 2.0 x 150 mm columns can be joined together in series to obtain double the column length. This setup produces similar resolution to that obtained using one 4.6 x 150 mm column, with the advantage of cutting solvent use to a third of that required for the larger diameter column. A single column can be used but the resolution is not as high.
• The method for N-glycan analysis is listed below.
• When starting the analysis bring the flow rate up slowly by running the startup method: N2x2-5m-Start-30%
• Follow this by running a complete gradient with no sample to condition the column with the running
method: N2x2-60m-Nlink-30%
• Run one of the following system suitability standards using the N2x2-60m-Nlink-30%
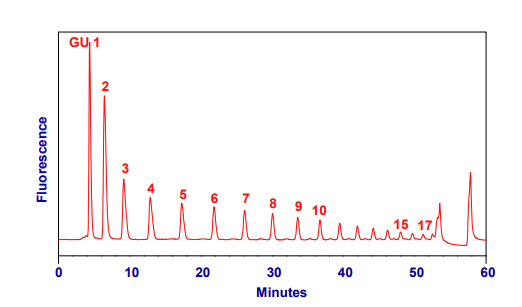
Figure 3: Typical HPLC chromatogram from 2-AB GHP run with method N2-30m-Nlink-35% on two 2 x 150 mm N2 columns in series.
Sample injected in 30%aqueous, 70% acetonitrile.
Glycan Analysis with two 2 x 150 mm N2 Amide HPLC Columns
Solvents
The glycan analysis gradients in this guide are based on the following solvents:
Solvent A : 50 mM ammonium formate pH 4.4,
(50 ml of product N-BUFFX40 diluted to 2 litres with water)
Solvent B : Acetonitrile
Gradient N2x2-5m-Start-30% : N-Glycan Analysis Startup
Use as a start up method for gradient for analysis of N-glycans which starts at 30% A.
Time (min) | % A | %B | 2 x 2mm column Flow Rate (ml/min) |
0 | 30 | 70 | 0.0 |
2 | 30 | 70 | 0.18 |
5 | 30 | 70 | 0.18 |
Gradient N2x2-30m-Nlink-30% : N-Glycan Analysis Gradient
Use as a gradient for analysis of N-glycans with GU between 4 and 12
Time (min) | % A | %B | 2 x 2mm column Flow Rate (ml/min) |
0 | 30 | 70 | 0.18 |
46 | 53 | 47 | 0.18 |
46.5 | 100 | 0 | 0.18 |
49.5 | 100 | 0 | 0.18 |
51 | 30 | 70 | 0.18 |
60 | 30 | 70 | 0.18 |
