Nicewicz Photoredox Catalysts for Anti-Markovnikov Alkene Hydrofunctionalization

INTRODUCTION
Although the Markovian rule plays a key role in explaining the mechanism of olefin Addition reaction, and it has also been widely used in scientific research and practical production, there are very few kinds of addition products with anti Markovian rules obtained through catalytic processes. In order to better support scientific research, Aladdin has provided a series of acridinium salts that can be photoinitiated. These salts can promote the hydrogenation functionalization of various olefins and have completely anti Markovian rule addition selectivity.
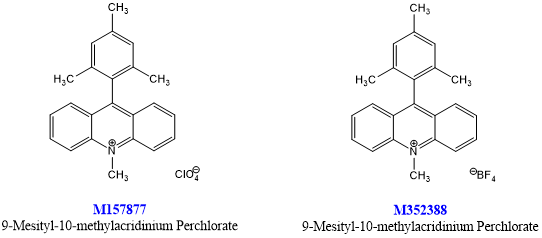
Cationic organic oxidant 9-methyl-10-methylacridine perchlorate was originally designed by Shunichi Fukuzimi's team.[1]Since its introduction, Nicewicz's group has demonstrated the widespread usefulness of this material and related photoreoxidation catalysts (M352388, M486765, M486763) when used together with thiolated hydrogen atom transfer catalysts.[2] This unique photoredox catalyst system enables various activated and unactivated olefins to hydrofunctionalize with various nucleophiles (such as carboxylic acids, amines, inorganic acids, propargyl alcohols and allyl alcohols) by generating olefin cation radical intermediates.[2-3]

ADVANTAGES
• Metal-free, visible light catalytic
• Stable in air and high humidity environment
• Mild reaction conditions
• The addition selectivity of anti-Markov rule
• Compared with Ru- and Ir- polypyridine photooxidants, it has superior oxidation capacity
• Acridine salt can be directly stimulated by LED floodlight irradiation
TYPICAL APPLICATIONS
Hydroetherification
Complete regioselectivity was observed in over the ratio of 20:1 of direct intramolecular anti-Markovnikov hydroetherification of alkenols when 9-mesityl-10-methylacridinium perchlorate was used with 2-phenylmalononitrile as a hydrogen atom donor.[4]

Hydroamination
Hydroamination of alkenes can be accessed using 9-mesityl-10-methylacridinium tetrafluoroborate (M352388), with complete anti-Markovnikov selectivity in the presence of a thiol-containing co-catalyst. With thiophenol, intramolecular hydroamination of unsaturated amines provided access to several nitrogen-containing heterocycles.[5]Furthermore, intermolecular hydroamination of aliphatic alkenes, α- and β-substituted styrenes, and heterocyclic amines were achieved with diphenyl disulfide.[6]
Hydrotrifluoromethylation
Diverse trifluoromethylated products have been obtained through this photoredox catalyst system. Single electron oxidation of the Langlois reagent (S464116) by 9-mesityl-10-methylacridinium tetrafluoroborate (M352388) and a thiol-containing co-catalyst (methyl thiosalicylate or thiophenol) results in the hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes with anti-Markovnikov selectivity. Nicewicz and co-workers provided 20 examples with 25–74% yields.[7]
Hydroacetoxylation
Anti-Markovnikov-selective hydroacetoxylation of styrenes, enamides, and trisubstituted aliphatic amines is possible with a range of carboxylic acids when using 9-mesityl-10-methylacridinium tetrafluoroborate (M352388) in combination with sodium benzene sulfinate or thiophenol. Seventeen reactions exhibited yields ranging from 29–99%.[8]
Addition of Mineral Acids
The anti-Markovnikov addition of strong Brønsted acids to alkenes is achieved with complete regioselectivity using these photocatalysts. For hydrochlorination, 9-mesityl-10-methylacridinium tetrafluoroborate (M352388) is used with thiophenol to generate the adduct. Hydrofluorination, hydrooxyphosphorylation and hydrooxysulfonylation are each effected by employing 9-mesityl-2,7-dimethyl-10-phenylacridinium tetrafluoroborate (M486765) with the hydrogen-atom donors 4-nitrophenyl disulfide or 4-methoxythiophenol. Here, the inclusion of 2,6-lutidinium salts were found to provide the best reactivity through a nucleophilic couterion.[9]
Intramolecular Hydrofunctionalization of Amides
The anti-Markovnikov intramolecular cyclization of unsaturated allylic amides and thioamides furnishes the resultant 2-oxazolines and 2-thiazolines, respectively. These transformations utilize a photoredox catalyst system comprising 9-mesityl-10-methylacridinium tetrafluoroborate (M352388) in combination with diphenyl disulfide. Through 17 examples, a range of 59–82% yields were observed.[10]
Hydrodecarboxylation
The anti-Markovnikov hydrodecarboxylation of carboxylic and malonic acids is accomplished with 9-mesityl-10-phenylacridinium tetrafluoroborate (M486763) and diphenyl disulfide. The decarboxylation reaction requires the inclusion of a base and a polar alcohol solvent, trifluoroethanol in order to afford good yields and scope. Eighteen examples were included to demonstrate the hydrodecarboxylation of primary, secondary, and tertiary carboxylic acids.[11]
Polar Radical Crossover Cycloadditions
In a tandem addition-cyclization sequence, polar radical crossover cycloadditions use variations of the photoredox catalyst system to generate the following products from alkenes: g-butyrolactones (with α,β-unsaturated carboxylic acids),[12] tetrahydrofurans (with allylic alcohols),[13] g-lactams (with unsaturated amides), and pyrrolidines (with unsaturated amines).[14]
Photooxygenation
Prior to Nicewicz’s reports, Griesbeck and Cho used 9-mesityl-10-methylacridinium perchlorate (M157877) in the presence of O2 as a visible-light mediated catalyst for Type II and electron-transfer photooxygenation reactions.[15]
References
1. Fukuzumi S, Kotani H, Ohkubo K, Ogo S, Tkachenko NV, Lemmetyinen H. 2004. Electron-Transfer State of 9-Mesityl-10-methylacridinium Ion with a Much Longer Lifetime and Higher Energy Than That of the Natural Photosynthetic Reaction Center. J. Am. Chem. Soc.. 126(6):1600-1601. https://doi.org/10.1021/ja038656q
2. Nicewicz D, Hamilton D. Organic Photoredox Catalysis as a General Strategy for Anti-Markovnikov Alkene Hydrofunctionalization. Synlett. 25(09):1191-1196. https://doi.org/10.1055/s-0033-1340738
3. Romero NA, Nicewicz DA. 2014. Mechanistic Insight into the Photoredox Catalysis of Anti-Markovnikov Alkene Hydrofunctionalization Reactions. J. Am. Chem. Soc.. 136(49):17024-17035. https://doi.org/10.1021/ja506228u
4. Hamilton DS, Nicewicz DA. 2012. Direct Catalytic Anti-Markovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc.. 134(45):18577-18580. https://doi.org/10.1021/ja309635w
5. Nguyen TM, Nicewicz DA. 2013. Anti-Markovnikov Hydroamination of Alkenes Catalyzed by an Organic Photoredox System. J. Am. Chem. Soc.. 135(26):9588-9591. https://doi.org/10.1021/ja4031616
6. Nguyen TM, Manohar N, Nicewicz DA. 2014. anti-Markovnikov Hydroamination of Alkenes Catalyzed by a Two-Component Organic Photoredox System: Direct Access to Phenethylamine Derivatives. Angew. Chem. Int. Ed.. 53(24):6198-6201. https://doi.org/10.1002/anie.201402443
7. Wilger DJ, Gesmundo NJ, Nicewicz DA. 2013. Catalytic hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes via an organic photoredox system. Chem. Sci.. 4(8):3160. https://doi.org/10.1039/c3sc51209f
8. Perkowski AJ, Nicewicz DA. 2013. Direct Catalytic Anti-Markovnikov Addition of Carboxylic Acids to Alkenes. J. Am. Chem. Soc.. 135(28):10334-10337. https://doi.org/10.1021/ja4057294
9. Wilger DJ, Grandjean JM, Lammert TR, Nicewicz DA. 2014. The direct anti-Markovnikov addition of mineral acids to styrenes. Nature Chem. 6(8):720-726. https://doi.org/10.1038/nchem.2000
10. Morse PD, Nicewicz DA. Divergent regioselectivity in photoredox-catalyzed hydrofunctionalization reactions of unsaturated amides and thioamides. Chem. Sci.. 6(1):270-274. https://doi.org/10.1039/c4sc02331e
11. Griffin JD, Zeller MA, Nicewicz DA. 2015. Hydrodecarboxylation of Carboxylic and Malonic Acid Derivatives via Organic Photoredox Catalysis: Substrate Scope and Mechanistic Insight. J. Am. Chem. Soc.. 137(35):11340-11348. https://doi.org/10.1021/jacs.5b07770
12. Zeller MA, Riener M, Nicewicz DA. 2014. Butyrolactone Synthesis via Polar Radical Crossover Cycloaddition Reactions: Diastereoselective Syntheses of Methylenolactocin and Protolichesterinic Acid. Org. Lett.. 16(18):4810-4813. https://doi.org/10.1021/ol5022993
13. Grandjean JM, Nicewicz DA. 2013. Synthesis of Highly Substituted Tetrahydrofurans by Catalytic Polar-Radical-Crossover Cycloadditions of Alkenes and Alkenols. Angew. Chem. Int. Ed.. 52(14):3967-3971. https://doi.org/10.1002/anie.201210111
14. Gesmundo NJ, Grandjean JM, Nicewicz DA. 2015. Amide and Amine Nucleophiles in Polar Radical Crossover Cycloadditions: Synthesis of ?-Lactams and Pyrrolidines. Org. Lett.. 17(5):1316-1319. https://doi.org/10.1021/acs.orglett.5b00316
15. Griesbeck AG, Cho M. 2007. 9-Mesityl-10-methylacridinium: An Efficient Type II and Electron-Transfer Photooxygenation Catalyst. Org. Lett.. 9(4):611-613. https://doi.org/10.1021/ol0628661










