Oligonucleotide synthesis
Over the years, oligonucleotide synthesis methods have evolved: from Michelson and Todd's early H-phosphonate and phosphoric acid triglyceride methods[1], and Khorana's phosphodiester method in the 1950s[2], to the revisiting of the phosphoric acid triglyceride method in the 1960s and the phosphite triglyceride method in the 1970s[3]. Each of these methods has its drawbacks. The phosphoramidite method, pioneered by Marvin Caruthers in the early 1980s and enhanced by the application of solid-phase techniques and automation, has become the method of choice.
The Phosphoramidite method
The synthesis of phosphoramidite oligonucleotides proceeds along the 3' to 5' direction (in contrast to the 5' to 3' direction of biosynthesis in DNA replication). One nucleotide is added per synthesis cycle [4].

Figure 1. The Phosphoramidite oligonucleotide synthesis cycle
The phosphoramidite monomer for DNA synthesis consists mainly of bases, deoxyribose, DMT at the 5' end and 2-cyanoethyl at the 3' end, and diisopropylamine groups. In addition, due to the presence of primary amino groups in adenine, cytosine and guanine and some artificial bases, it is also necessary to protect them with appropriate protecting groups in the unit. The DMT protecting group must be removed from the carrier-bound nucleoside prior to oligonucleotide synthesis.
Step 1: Activation and coupling
Prior to coupling, the activator tetrazole provides a proton to the nitrogen atom of the diisopropylamine group on the 3' phosphoric acid of the phosphoramidite monomer. The protonated diisopropylamine is a good leaving group to form a reactive intermediate like phosphoramidite tetrazole with the tetrazole, a process known as activation. The deprotected 5'-end -OH will couple with the phosphoramidotetrazole active intermediate to form a new phosphorus-oxygen bond and remove the tetrazole. In this step the nucleotide chain is extended. It is important to note that: excess water in the monomer and activator leads to low coupling efficiency, and more impurities in the raw material also lead to low coupling efficiency.

Figure 2. Phosphoramidite coupling reaction mechanism
Nucleoside phosphoramidites are reasonably stable in an inert atmosphere and can be prepared in large quantities, shipped around the world and stored as dry solids for several months prior to use. Only upon protonation do nucleoside phosphoramidites become reactive.
Step 2: Capping
It is not unreasonable to expect a yield of 99.5% during each coupling step, but even with the most efficient chemistry and the purest reagents it is not possible to achieve 100% reaction of the support-bound nucleoside with the incoming phosphoramidite. This means that there will be a few unreacted 5'-hydroxyl groups on the resin-bound nucleotide; if left unchecked, these 5'-hydroxyl groups would be available to partake in the next coupling step, reacting with the incoming phosphoramidite. The resulting oligonucleotide would lack one base, and would correspond to a deletion mutation of the desired oligo. If such deletion mutations were left unchecked they would accumulate with each successive cycle, and the final product would be a complex mixture of oligonucleotides, most of which would carry incorrect genetic information, and which would be difficult to purify. This would ruin any subsequent biochemical experiment.

Figure 3. Deletion mutationSequence of the correct oligonucleotide (top); and a failure sequence (bottom) containing a deletion mutation, corresponding to the deletion of the thymine base at position 6.
Deletion mutations are avoided by introducing a "capping" step after the coupling reaction, to block the unreacted 5'-hydroxyl groups. Two capping solutions are used on the synthesizer: acetic anhydride and N-methylimidazole (NMI). These two reagents (dissolved in tetrahydrofuran with the addition of a small quantity of pyridine) are mixed on the DNA synthesizer prior to delivery to the synthesis column. The electrophilic mixture rapidly acetylates alcohols, and the pyridine ensures that the pH remains basic to prevent detritylation of the nucleoside phosphoramidite by the acetic acid formed by reaction of acetic anhydride with NMI. Acetylation of the 5'-hydroxyl groups renders them inert to subsequent reactions.

Figure 4. Mechanism of the cap addition step in phosphoramidite oligonucleotide synthesis
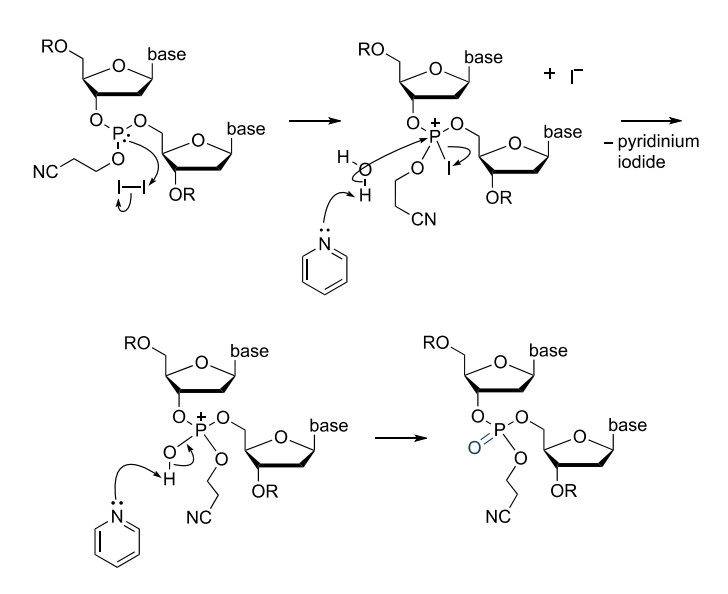
Step 3: Oxidation
The phosphite-triester (P(III)) formed in the coupling step is unstable to acid and must be converted to a stable (P(v)) species prior to the next TCA detritylation step. This is achieved by iodine oxidation in the presence of water and pyridine. The resultant phosphotriester is effectively a DNA backbone protected with a 2-cyanoethyl group. The cyanoethyl group prevents undesirable reactions at phosphorus during subsequent synthesis cycles.
Figure 5. Mechanism of the oxidation step in phosphoramidite oligonucleotide synthesis
On some DNA synthesizers there is a second capping step after iodine oxidation. The purpose of this is to dry the resin, as residual water from the oxidation mixture can persist and inhibit the next coupling reaction. The excess water reacts with the acylating agent to form acetic acid which is washed away in the THF/pyridine solvent mixture.
Step 4: Detritylation
After phosphoramidite coupling, capping and oxidation, the DMT protecting group at the 5' end of the nucleotide must be removed so that the primary hydroxyl group can undergo a coupling reaction with the next nucleotide phosphoramidite. Deprotection with trichloroacetic acid in dichloromethane is rapid and quantitative. Note that too concentrated a TCA solution or too long a depyrogenation time will result in depurination and thus reduce the overall yield of the final oligonucleotide. The cleavage produces a DMT carbon cation showing an orange color, which has visible light absorption at 495 nm and the coupling efficiency can be monitored by measuring its absorbance. After this step, the extended oligonucleotide strand goes to the next cycle, and then the cycle is repeated until the strand is extended to the desired length.
References:
1. A. M. Michelson,Alexander R. Todd. Nucleotides part XXXII. Synthesis of a dithymidine dinucleotide containing a 3′: 5′-internucleotidic linkage[J]. Journal of the Chemical Society (Resumed),1955,0(0). https://doi.org/10.1039/JR9550002632
3. Reese Colin B. The chemical synthesis of oligo-and poly-nucleotides by the phosphotriester approach[J]. Tetrahedron,1978,34(21). https://doi.org/10.1016/0040-4020(78)87013-64. 4.Beaucage S.L.,Caruthers M.H.. Deoxynucleoside phosphoramidites
--A new class of key intermediates for deoxypolynucleotide synthesis[J]. Tetrahedron Letters,1981,22(20). https://doi.org/ 10.1016/S0040-4039(01)90461-7
Aladdin:https://www.aladdinsci.com
List of related products