PD-1/PD-L1 Signaling Pathway

Introduction
Programmed cell death protein 1, also known as PD-1 and CD279, is a cell surface receptor that plays an important role in downregulating the immune system and promoting self-tolerance by inhibiting T cell inflammatory activity. In humans, it is encoded by the PDCD1 gene. PD-1 is a member of the immunoglobulin superfamily and is expressed on t cells and preB cells. PD-1, as a receptor, has two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC). When PD-1 binds to the ligand, it induces the dual mechanism of apoptosis of antigen-specific t cells in lymph nodes (programmed cell death), while reducing the apoptosis of regulatory t cells (anti-inflammatory and inhibitory t cells).
Based on previous studies, we found that PD-L1 is responsible for tumor immune regulation. This is because the binding affinity of PD-1 with PD-L1 is three times that of PD-1 for the expression of PD-L2 and PD-L1 in tumor and hematopoietic cells, which is determined by the stimulation of pro-inflammatory factors such as IFN-γ and TNF-α. PD-L1 is widely expressed in hematopoietic and non-hematopoietic cells, while PD-L2 is limited in macrophages, dendritic cells (dc), and mast cells that secrete IL-4 and IFN-γ. PD-L2 has recently been reported to interact with macrophage (M8) protein's rejection guide molecule B (RGMB). Although there have been some reports on PD-L2, there is little information about its role in cancer immunosuppression.
Mechanism of PD-1and PD-L1/L2 mediated immune resistance
The PD-1/PD-L signaling pathway regulates the induction and maintenance of peripheral tolerance. PD-L can induce T cell tolerance through its expression in drug-resistant dendritic cells. In addition, PD-L1 is constitutively expressed on APCs and dendritic cells and further upregulated by pro-inflammatory cytokines. When T cells are activated, PD-L1 is up-regulated. After initial T cell activation, PD-1/PD-L interactions can limit self-reactive T cell proliferation and cytokine production.
In the tumor microenvironment, PD-1 and its ligand, PD-L1, evade tumor neutralization and immune surveillance and play an important role in tumor progression and survival. Studies have shown that PD-1 is expressed on a variety of immune cells, tumor-infiltrating lymphocytes (TILs), and tumor cells. The binding of PD-L1 to T cell PD-1 leads to T cell dysfunction, failure, neutralization, and the production of interleukin-10 (IL-10) in tumor masses. Therefore, the function of tumor overuse of PD-L1 is to protect itself from cytotoxic T cells (CD8+) mediated cell killing. Due to depletion of CD8+ T cells, tumor cells become very aggressive and secrete several pro-inflammatory cytokines. These cytokines further upregulate PD-L1. Another subtype of T cells, such as regulatory T cells (Treg, CD4+ Foxp3+), create a highly immunosuppressive tumor environment by maintaining PD-1 expression on their surface. We observed that in the presence of CD3 and TGF-β, PD-1 receptors in regulatory T cells (Treg cells) increased the neonatal transformation of original CD4+ T cells into Treg cells, thereby attenuating the immune response. This transformation increases Treg expression and CD4+ T cell immunosuppressive function by inhibiting the mammalian target of rapamycin (mTOR) -Akt signaling cascade. In conclusion, PD-1 expression not only inhibited the function of effector t cells, but also enhanced the transformation of immunosuppressive Treg cell population.
Although PD-1 has been extensively studied in t cells, its function in b cells has also become apparent in tumor immunosuppression. The expression of PD-1 is highly regulated during B cell differentiation, but the expression of PD-1 in pro-B cells is not significant and increases with B cell differentiation. In addition, PD-1-activated toll-like receptor 9 (TLR9) agonists significantly promoted the maturation of b cells. Thus, inhibiting PD-1 function on B cells has been shown to enhance antigen-specific antibody responses, suggesting that PD-1 plays a role in inhibiting B-cell-mediated t cell activation.
Cancer immunotherapy
In cancer immunotherapy, antigen presenting cells (APCs) bind to the antigen (Ag) released by tumor cells and T cells, activating T cell receptor (TCR) and MHC binding. Tumor interstitial PD-L1 interacts with T cell PD-1 to inhibit T-cell-mediated tumor cytotoxicity. If anti-PD-1 and PD-L1 drugs are used, blocking PD-1 or PD-L1 can increase T-cell-mediated tumor cytotoxicity and eventually kill tumor cells.
Unlike early activated CTLA-4, the PD-1 checkpoint only regulates the activity of cytotoxic T lymphocytes to migrate to the tumor. The expression of PD-L1 ligand was selective and not overexpressed in normal inflammatory tissues. This makes it possible to block the biological effects of drugs in the PD-1 pathway, which are much less toxic than anti-CTLA-4. The first small trial of nivolumab, an anti-PD-1 drug, was very successful. In subsequent clinical trials, tumor regression was also observed in a significant proportion of refractory melanoma, kidney cancer, and lung cancer. Lung cancers that were previously considered non-immunogenic (unable to cause immune rejection) have been shown to be resistant to PD-1. The discovery immediately changed the landscape of PD-1 applications, and researchers conducted multiple clinical trials of anti-PD-1 and anti-PD-L1 drugs in various cancer types. Since anti-PD-1 monoclonal antibodies against advanced melanoma were first approved in early 2014, six different drugs have entered the clinic for a variety of indications. These indications include non-small cell lung cancer, Hodgkin's lymphoma and cutaneous Meckel cell carcinoma, kidney cancer, bladder cancer, head and neck cancer, and tumors with a high mutation burden for a genetic marker known as microsatellite instability. This suggests that anti-PD-1 and anti-PD-L1 drugs may act as broad-spectrum anti-tumor agents in tumors expressing PD-L1.
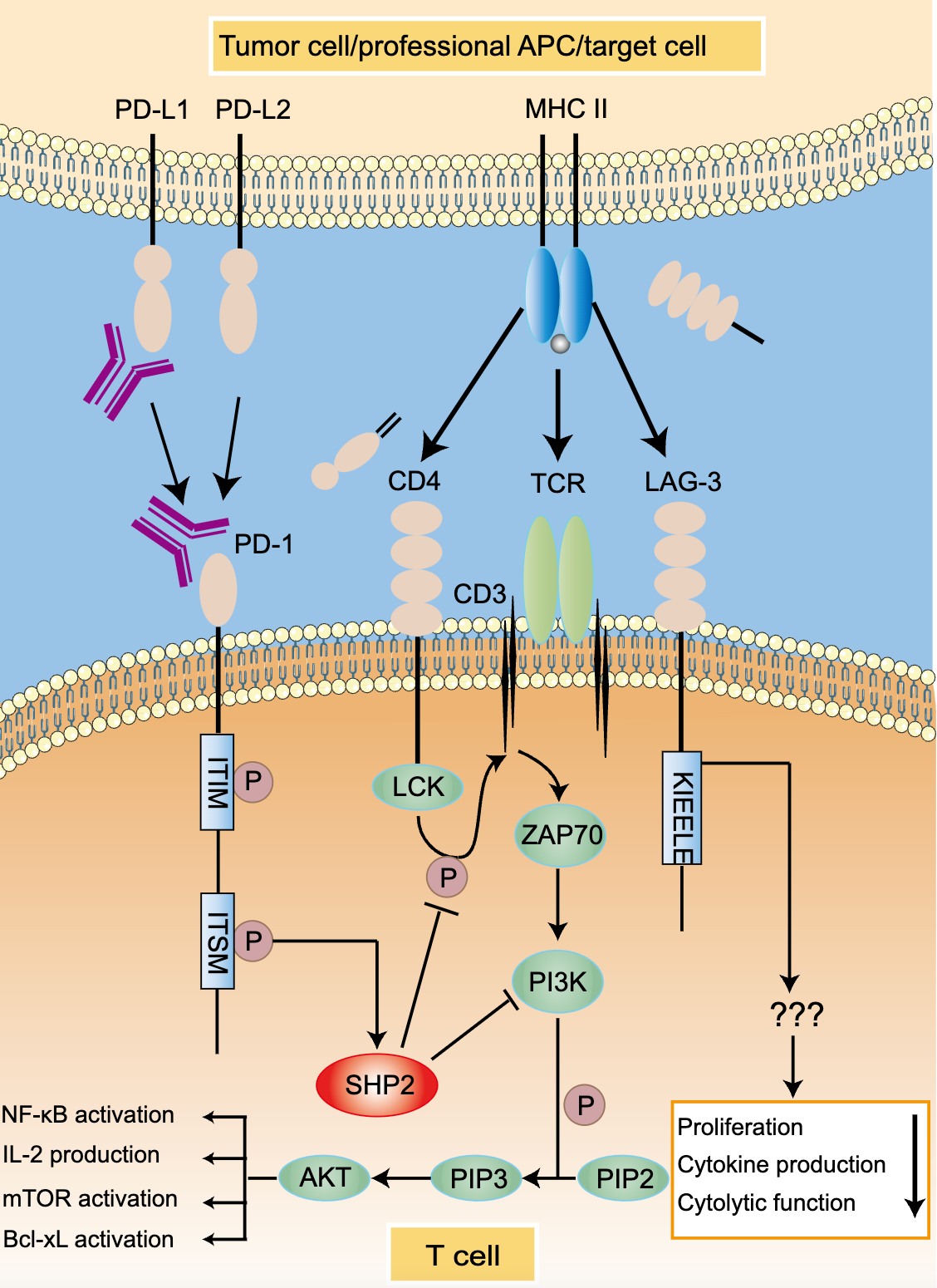
Figure.1 PD-1/PD-L1 signaing pathway
Reference
1. Francisco L M., et al. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews.2010, 236: 219–42.
2. Arlene H S., et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. NATURE IMMUNOLOGY. 2007, 8: 239-245.
3. Hashem O A., et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Frontiers in Pharmacology. 2017, 8: 1-15.
