Preparation and functionalized design of novel graphene-based nanostructures

Introduction
At its inception, graphene was essentially a game for physicists. It was difficult to synthesize sufficient quantities of high-quality graphene, and it was not until researchers prepared monolayer graphene by simple mechanical peeling of graphite using tape that this new material with excellent electronic properties became known. In the decade since the successful preparation of monolayer graphene, graphene has gained tremendous attention among the general public, becoming the most "talked about" material on online social media and widely considered to be the new miracle material 1. However, key issues that have prevented the widespread use of graphene remain, including poor processability and, for electronics, the lack of band gaps in its electronic structure.
In recent years, chemists have learned how to "play" with this unique material by enhancing its processability and versatility and developing different strategies to functionalize and process it. The production of graphene starting from cheap graphite has been demonstrated on a large scale2and the growth of high quality graphene on metal substrates or silicon carbide has been optimized, with very good progress in different fields of application3,4.
A number of previous research efforts have described how the unique two- dimensional (2D) shape of graphene gives it unique properties5 such as its interaction with organic molecules in vacuum, liquid and solid films6 . Using chemical techniques, it is also possible to effectively tailor this insoluble and almost chemically inert material into a variety of forms, from simple soluble flakes to layered structures in which 2D graphene flakes are assembled into three-dimensional (3D) bulk materials or foams with very promising applications in electronics, composites, energy storage or catalysis.
Chemical properties of monolithic graphene dispersions
The simplest way to modify or process graphene is to treat single sheets of graphene in solution. Large-scale production can be achieved in two ways: one does not use non-covalent supramolecular interactions that disrupt the graphene lattice 7-9, and the reaction conditions are milder. The other is to turn graphene into graphene oxide (GO) and then reduce it. This approach requires more demanding reaction conditions and inevitably leads to chemical defects on the sheet surface (Figure 1) 10-12.

Figure 1: Schematic diagram of different methods for removing graphene layers and functionalized graphene using covalent or non-covalent (supramolecular) methods
GOs (Item No.G405797,G139812) have not received much attention relative to other members of the graphene family due to their chemical defects that weaken many of the unique physical properties of graphene. However, these properties also allow GOs to have better processability, versatility and water solubility. As for their electrical conductivity, it can be re-established by thermal, chemical or electrochemical reduction. However, some damage to the lattice is always present, which makes reduced graphene oxide (RGO) (Item No.G196551) to perform much less well than graphene. Nevertheless, different forms of graphene oxide and reduced graphene oxide are still being produced and successfully applied 13-20, some of which seem to be closer to commercial applications than most advertised graphene in the field of electronic or transparent conductors.
Exfoliation of graphite (Item No.P196402,E196403) produces graphene as a nano- and meso-porous complex phenomena on the scale. Similar to other solubilization processes, this process relies on a complex competition between chemical, electrostatic and van der Waals interactions 21, and fluid dynamics at the microscopic scale. Recently, a systematic and comparative study of exfoliated graphene produced using mechanical, chemical and electrochemical methods has been carried out by researchers 22 Of these three methods, chemical oxidation to produce graphene oxide has proven to be a very effective but destructive method for exfoliating graphite. Electrochemical oxidation, which uses an electric field to drive molecular intercalation in graphite, allows for rapid exfoliation and deep destruction of large amounts of graphite, but also causes damage to the resulting graphene lattice.
Supramolecular exfoliation methods based on the use of sonication in organic solvents yield the best quality graphene, but the obtained flakes have a low lateral size (usually <1 μm) due to the influence of the energy transfer of the sonication process. The differences observed from the three methods suggest that there is a trade-off between the speed of exfoliation, the efficiency and the quality of the preserved material 22.
Once exfoliated in solution, graphene flakes can be chemically modified (in covalent or non-covalent form) using a variety of molecular or nano-objects to yield new, customized graphene-based materials 23. The carbon structure of this highly conjugated sp2 graphene gives it a strong affinity for polyaromatic organic materials, such as organic semiconductors or industrial dyes, which are characterized by extended π- electron leaving domains. These molecules, unlike graphene, have excellent processing properties and tunable HOMO, LUMO, and energy band gaps.6
This affinity is being investigated to create organic hybrids of graphene with good solubility in water or organic solvents, good dispersion in polymer composites, or novel optoelectronic functionality. Although it is difficult to provide an unbiased and exhaustive overview of all graphene-based hybridization systems developed so far, there are still many interesting examples, including hybridization based on organic materials, as well as metal nanoparticles and biomolecules, even DNA. the most commonly used part is perylene (Item No.P106993) 9,24,25, pyrene (Item No.R10905,R109056,P141124) 8,26-29, porphyrins (Item No.T304418,P342370,P103197)30-34, fullerenes (Item No.F491245,F117656,F141063) 35,oligothiophenes 36,37, polythiophenes 38,39, metal nanoparticles 40,41, and even biomolecules 42,43.
Several empirical models have been developed to predict the dispersion of graphene in different solvents using the surface tension or solubility parameters of the solvent 7. However, it is still not clearly understood at the molecular level why some molecules interact more effectively with graphene than others. Researchers conducted a systematic study comparing several pyrene sulfonate sodium salts (Item No.P131259,D304268) in terms of their efficiency. Water-soluble pyrene moieties, as stripping agents, were used to prepare stable aqueous suspensions using a liquid stripping method 8. These pH-sensitive derivatives are not only of great interest for basic research, but also possess practical applications in themselves due to their high photoluminescence quantum yields, excellent water solubility and non-toxicity. They have been used commercially on a large scale in highlighters, pencils and soaps.
Related researchers examined a series of four pyrene derivatives with increasingly polar groups and found that they all exfoliate graphite to produce stable graphene suspensions in water. Thin monolayers of graphene covered by a layer of pyrene molecules of corresponding proportions were obtained 26. The total concentration of the suspended graphene depends strongly on the number of polar groups on the hydrophobic pyrene nucleus. Molecular dynamics calculations show that a key factor in the interaction of pyrene derivatives with graphene involves a thin layer of solvent molecules, which is confined between the pyrene molecules and the graphene surface. For molecules with an asymmetric shape displaying a strong dipole, the amphiphilic pyrene sulfonate molecule changes its orientation as it approaches the surface and slips into this layer in a most efficient manner. The simulations show that the molecular dipole itself is not important, but it facilitates the "sliding" of the molecule into the solvent layer, resulting in a lateral displacement of the water molecule between the aromatic nucleus of the pyrene dye and the graphene substrate.
The interaction of organic molecules with graphene sheets at the nanoscale has been fundamentally revealing not only for a better understanding and improvement of graphene exfoliation8 , but also for its advanced applications. Thus, pyrene-based methods for exfoliating graphite with well-established industrial dyes have been extended to process graphene in polymers45 . Similarly, pyrene sulfonic acid has shown similar ability to solubilize other two-dimensional materials such as boron nitride (Project no.B106032,B299204,B299318), tungsten disulfide (Project No.T196561,T137743), molybdenum sulfide (Item No.T107763), molybdenum sulfide (Project No.M336538), selenides and tellurides (Figure 2). These materials can be used to fabricate photodetector devices based on different layers of two-dimensional materials44 .

Figure 2: A) Comparison of the chemical molecular formulae of four pyrene derivatives used for supramolecular graphite exfoliation; B) Optical images of two- dimensional crystal-based dispersions prepared using pyrene derivatives (numbers 1, 2, 3 and 4 refer to the number of sulfonic acid groups of the organic dyes shown in (A) 8,44.
Along with non-covalent modification methods based on supramolecular interactions, different synthetic methods have been proposed worldwide to covalently bind functional organic materials to graphene and graphene oxide. Developing more efficient and controllable synthesis protocols to achieve this goal is a challenging problem. Given its poor dispersion and inherently inert chemical structure, graphene has a much more limited reactivity than graphene oxide, mostly relying mainly on the formation of covalent bonds between free radicals or amphiphilic molecules and C=C bonds 46,47. In contrast, graphene oxide can undergo the typical reactions of carboxyl, carbonyl, epoxy and hydroxyl functional groups. Therefore, it is easier to modify than graphene. The most common synthesis method for graphene oxide functionalization is to activate carboxyl groups and then react with nucleophilic groups 37 (i.e. graphene oxide functionalization). Recently, an alternative approach to graphene oxide functionalization has been proposed, which relies on MW irradiation to promote the silylation of surface oxygens. This method can be used to graft tri alkoxy silane-terminated π-conjugated chromophores on the surface of graphene oxide flakes 36.
Several authors have investigated how the properties of functional molecular materials affect the behavior of graphene after physical doping 48-50 or covalent bond grafting 37,51. Meanwhile, the question of how the presence of graphene would affect the properties of the molecule remains. It is known that graphene and graphene oxide burst the photoelectric emission of luminescent molecules 52-54, but to what extent is this interaction affected by the nanoscale structure of the graphene-organic combination? By designing the structure of the system at the nanoscale, it is possible to tune this interaction. For example, low bursts 37 (~16%) are observed when low- sulfothiophene dyes are tethered to graphene oxide using flexible alkyl linkers, and stronger bursts (~60%) are achieved with shorter linkers 51, and almost complete quenching can be achieved by depositing graphene oxide on a single molecular layer self-assembled from the same low-sulfothiophene 52, (see Figure 3).
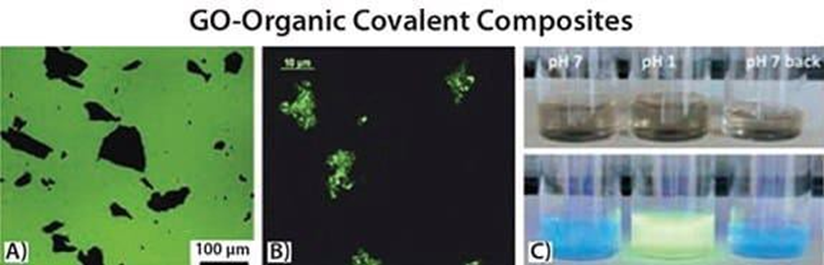
Figure 3: A) Thin fluorescence of graphene oxide flakes on SiOx bursting oligothiophene molecules52 ; B) Fluorescent graphene oxide flakes with flexible diamine linker, functionalized with oligothiophene dye to obtain37 ; C) pH neutral graphene oxide- oligothiophene covalent composites under HCl acidification and triethylamine (TEA) reneutralization (from left to right) under normal light (top ) and UV irradiation (bottom) images showing the reversible emission switch.
In addition to fluorescence bursts, the same oligothiophene-graphene oxide tethers have been used to study how the tethering of molecules to graphene oxide affects their chemical-physical properties. The molecules behave differently when they are separated into a solvent rather than being tethered to a bulk macroscopic surface. However, molecules tethered to graphene or graphene oxide are in an ambiguous state because the size of the two-dimensional sheet is much larger than the size of a typical molecule. In fact, this is more similar to the case of attaching to a large conventional surface, but simultaneously dissolving in solution 5. We investigated the interaction of graphene oxide with terthiophene dyes using pH-sensitive fluorescence emission to determine how the emission changes when the molecule is free in solution or is tethered to graphene oxide (Figure 3B-C). We found that covalent attachment of graphene oxide does not disrupt the absorption and emission properties of the dye, especially the pH sensitivity.
From solution to real material
Once two-dimensional graphene sheets are functionalized in solution, it is very easy to deposit them with micro/nano electrodes on silicon substrates to create devices such as transistors or sensors. Using the high processability and easy charge transfer between graphene and organic materials, devices with tunable percolation properties can be prepared, allowing them to combine the good charge mobility of RGO with the semiconductor behavior of organic materials.
Some examples already available include RGO/poly(3-hexylthiophene) bilayers 18, graphene/phenyloctane and graphene/arachidic acid blends 55, and blends of graphene with high-performance semiconductor polymers such as poly[N,N-9-bis(2-octyldodecyl)-naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl]-alt-5,59-(2,29-bis thiophene)] and (P(NDI2OD-T2)) 56. Graphene and organics can be deposited simultaneously to form a dense copolymer 55,56, or sequentially to form a bilayer 18.
Using the ability of organic π-conjugated molecules to remove graphite layers 8,55, graphene exfoliation can be performed in a solution used for organic material deposition. Alternatively, graphite can be exfoliated first to obtain graphene, which is subsequently mixed with organic semiconductors 56. By using a suitable solvent and applying an electric field, the flakes can be selectively deposited on the desired substrate 57,58.
Processing graphene in a more complex multilayer structure
In addition to simple mixtures or bilayers containing semiconductor polymers, more complex structures can be constructed using the enhanced processability and tunability of chemically modified graphene.
The multilayer "sandwich" structure allows the formation of self-assembled single molecule layers of 1-pyrenyldibutyric acid n-hydroxy succinimide by alternate layer-by-layer (LbL) deposition using high quality graphene. Due to the doping of graphene with pyrene, the conductivity of this multilayer structure is enhanced by 6 orders of magnitude compared to pure graphene59. In a similar approach, a multilayer structure of GO alternating with poly(lysine) can be created using LbL. Heated to 800 °C, GO is reduced and doped with poly lysine, yielding miniature supercapacitors with ultra- high-volume capacitance of ~488 F/cm3 and excellent rate capability of up to 2000 V/s.
Two-dimensional porous structures based on GO can be obtained by covalent functionalization using linkers of different lengths. For example, the p-hydroxy ethylene diamine intercalation reaction of graphene oxide (GO) is a convenient and simple method to prepare graphene-based materials in the form of flakes 60. The generation of this constrained system (covalent "stitching") along the c-axis of graphene allows for a significant change in the interlayer distance compared to pristine graphene oxide, thanks to the intercalation of p-hydroxy diamine (Item No.P108424) cross-linking process with the epoxide surface groups of the adjacent graphene oxide flakes. The increased interlayer distance of graphene oxide allows to increase the porosity and specific surface area of the material by a factor of 5 over pristine graphene oxide.
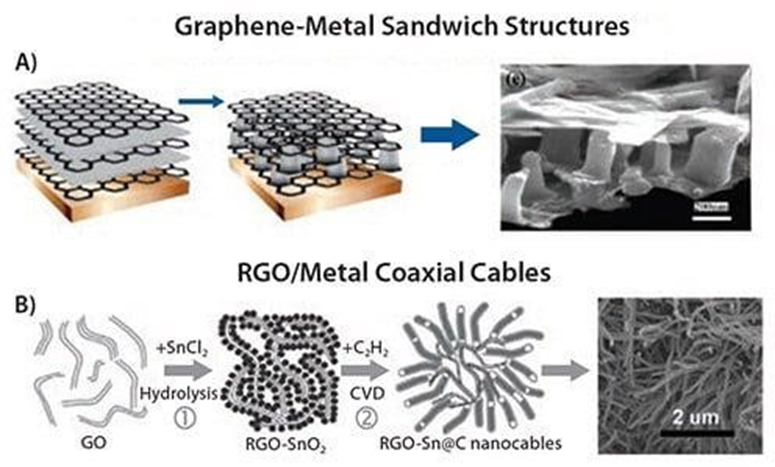
A coarser-grained structure was obtained, forming multiple layers of solvent- exfoliated graphene sheets and thin layers of vacuum-sublimated metallic tin. After annealing at 300 °C in argon/hydrogen, the tin layers decomposed into isolated nanopillars that act as spacers for graphene and ion reservoirs for lithium storage in batteries (Figure 4A) 61.
The formed 3D multilayer nanostructures are directly used as anode materials for rechargeable Li-ion batteries without the addition of any polymer binder or carbon black. Electrochemical measurements show very high reversible capacity and excellent cycling performance of the material at current densities up to 5Ag-1. This is a good example of how liquid processing can be combined with vacuum-based techniques, such as thermal evaporation, to obtain novel structures. In another example, highly homogeneous graphene oxide flake interlayers embedded with porous carbon (PC) were obtained using ionic liquid functionalized graphene oxide flakes as shape guides and resorcinol/formaldehyde polymers as carbon precursors.
The obtained PC/GO/PC multilayer sandwich structure shows excellent rate capability with a high specific capacitance of 341 Fg-1 at 5 mVs-1 and good cycling stability of more than 35,000 cycles without any conductive additives.
Graphene composites are produced as monolayers in solution or as very thin layers on planar substrates, primarily for electronic applications. Moving to larger scales, more complex structures can be built by using mesoscopic templates to obtain large, macroscopic layered materials.
Macroscale materials: foams for energy storage or catalysis
Through graphene oxide oxidation, reduction and/or chemical functionalization experiments, different kinds of 2D flakes can be processed and even grown directly on almost any micro- or nanopore template, such as metal templates 62,63 or polymeric foam materials 64, inorganic zinc oxide nanocrystals 65, or water-organic-liquid interfaces in emulsions 66,67. Graphene can be used to wrap or encapsulate other nanostructures 68 or even electrodes 58.Conversely, its surface can be coated with other materials as 2D substrates to support less conductive or less robust materials 69,71.
These structures are often visually beautiful (Figures 4-5), but they are also interesting from a technical point of view because they can be further functionalized by other materials that have synergistic and complementary properties to graphene.
Figure 4: A) Schematic diagram of the graphene/Sn-nanopore nanostructure preparation process and SEM images 61; B) Schematic diagram of the synthesis steps of RGO-loaded Sn/C nanowires, and the corresponding SEM images
Figure 5: Graphene-based 3D structure templates using A) metal foam 62; B) polymeric foam 64, or C-E) liquid emulsion 66
Graphene has excellent electrical, optical, mechanical and thermal properties, but also has some inevitable drawbacks. Other materials have better performance in energy applications; Fe2O3 is a low-cost, abundant and non-toxic capacitive electrode material with a high theoretical capacity (1007 mAhg-1, much better than the commonly used graphite-based material (372 mAhg-1)). However, the practical application of Fe2O3 based electrodes is limited due to their low conductivity and poor cycling stability. Therefore, the combination of graphene and Fe2O3 has comparative advantages for the development of next-generation batteries.
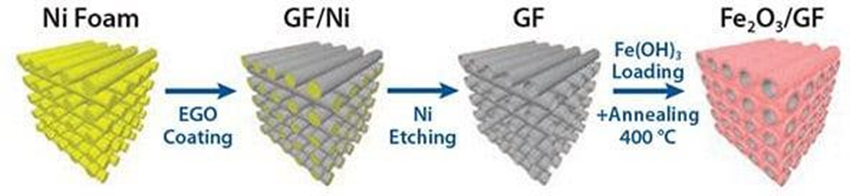
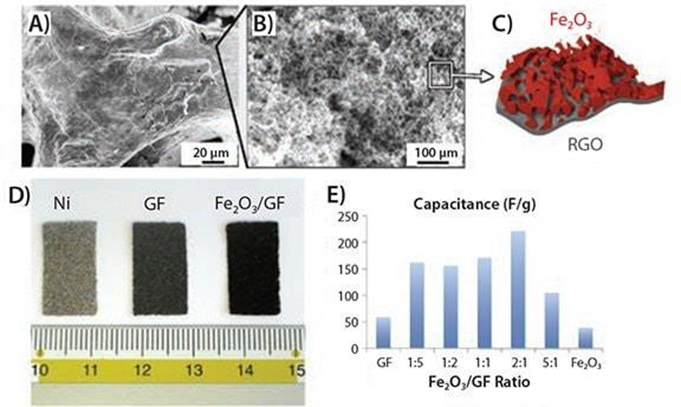
The authors used a combination of electrochemical and solution processing techniques to produce layered Fe2O3 based graphene composites for energy storage. Electrochemically exfoliated graphene oxide (EGO) flakes were produced using a custom-built device that allowed rapid expansion of the graphite flakes and efficient exfoliation of the expanded graphite via an electrochemical route. The generated flakes were deposited on sacrificial nickel foam along with a colloidal precursor of iron hydroxide. The subsequent calcination treatment simultaneously conducts the EGO foam and converts Fe(OH)3 to hematite (α-Fe2O3), producing a nano-Fe2O3 layer on the surface of the mesoporous EGO foam. The resulting graphene/metal oxide hybrid is a continuous, conductive 3D composite that is an ideal lithium storage structure (Figure 6) with a layered meso-nano porous structure (Figure 7A-D). By using liquid processing, the nanostructured material can be optimized by adjusting the ratio of Fe2O3: EGO in order to maximize its performance as a standard coin cell electrode 63.
With the decoration of Fe2O3 nano porous coating, the initial discharge capacity can be increased to 701 mAhg-1, a value comparable to that of commercially available batteries, and a high energy capacity can be maintained after the first discharge/charge cycle (Figure 7E).
Figure 6: Schematic diagram of template-assisted deposition of electrochemically exfoliated graphene oxide (EGO) on nickel metal foam and successive reduction to obtain conductive foam (GF) either uncoated or coated with Fe2O3(α- Fe2O3)
Figure 7: A) SEM image of a mesoporous conductive graphene foam coated with nano porous Fe2O3 layers; B) inset of Figure A; C) inset showing the hierarchy of Fe2O3/GF porous layers; D) comparison of photos of pristine Ni foam, GF and Fe2O3/GF samples; E) specific capacitance values obtained for different Fe2O3: GF ratios 63.
In addition to energy storage, catalysis is another promising area where graphene- based porous structures can be used. In particular, graphene-based materials have shown promise as efficient cathode catalysts for oxygen reduction reactions (ORR), a key process in fuel cells.
The ORR of Fe3O4/graphene foam in alkaline media shows a more aggressive onset potential, higher cathodic density and higher electron transfer number compared to Fe3O4 nanoparticles supported on more conventional materials (N-doped carbon black or N-doped graphene sheets). This improvement can be attributed to the role of three-dimensional large pores and the high specific surface area of the graphene-based support in improving the ORR performance 69. A more detailed review of porous graphene materials for advanced electrochemical energy storage and conversion is available in the literature 72.
Conclusion
Graphene chemical exfoliation, processing and functionalization directions are currently booming, with several scientific results published every month. This trend is expected to accelerate in the coming years as graphene with standardized quality, well-controlled properties and low cost will become more widely available for industrial use around the world. Multilayer graphene powders are already affordable, and applications using graphene-based composites are already entering the end-user market. In addition, chemical functionalization can enhance the interaction of graphene with the polymer matrix in composites to improve mechanical properties.
Not only does graphene have excellent electronic properties, its chemical versatility and processability also enable it to produce a wide range of materials with advanced and unprecedented functionality. Indeed, the field of chemistry itself is experiencing a graphene-spurred renaissance. Conventional organic chemistry methods need to be properly retooled to accommodate graphene tailoring, which is a challenging problem for organic chemists. At the same time, new reactions have to be developed in order to realize different composites: from simple graphene sheets wrapped by thin molecular layers, to bulk composites of graphene and organic materials, to more complex forms where graphene acts as a 3D, robust and versatile scaffold that can be combined with organic, inorganic and even biological materials.
References
1.Sechi G, Bedognetti D, Sgarrella F, Eperen LV, Marincola FM, Bianco A, Delogu LG. 2014. The perception of nanotechnology and nanomedicine: a worldwide social media study. Nanomedicine. 9(10):1475-1486. https://doi.org/10.2217/nnm.14.78
2.Paton KR, Varrla E, Backes C, Smith RJ, Khan U, O?Neill A, Boland C, Lotya M, Istrate OM, King P, et al. 2014. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nature Mater. 13(6):624-630. https://doi.org/10.1038/nmat3944
3.Novoselov KS, Fal?ko VI, Colombo L, Gellert PR, Schwab MG, Kim K. 2012. A roadmap for graphene. Nature. 490(7419):192-200. https://doi.org/10.1038/nature11458
4.Ferrari AC, Bonaccorso F, Fal'ko V, Novoselov KS, Roche S, Bøggild P, Borini S, Koppens FHL, Palermo V, Pugno N, et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 7(11):4598-4810. https://doi.org/10.1039/c4nr01600a
5.Palermo V. 2013. Not a molecule, not a polymer, not a substrate? the many faces of graphene as a chemical platform. Chem. Commun.. 49(28):2848. https://doi.org/10.1039/c3cc37474b
6.Schlierf A, Samorì P, Palermo V. 2014. Graphene?organic composites for electronics: optical and electronic interactions in vacuum, liquids and thin solid films. J. Mater. Chem. C. 2(17):3129. https://doi.org/10.1039/c3tc32153c
7.Coleman JN. 2013. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res.. 46(1):14-22. https://doi.org/10.1021/ar300009f
8.Schlierf A, Yang H, Gebremedhn E, Treossi E, Ortolani L, Chen L, Minoia A, Morandi V, Samorì P, Casiraghi C, et al. 2013. Nanoscale insight into the exfoliation mechanism of graphene with organic dyes: effect of charge, dipole and molecular structure. Nanoscale. 5(10):4205. https://doi.org/10.1039/c3nr00258f
9.Kozhemyakina NV, Englert JM, Yang G, Spiecker E, Schmidt CD, Hauke F, Hirsch A. 2010. Non-Covalent Chemistry of Graphene: Electronic Communication with Dendronized Perylene Bisimides. Adv. Mater. 22(48):5483-5487. https://doi.org/10.1002/adma.201003206
10.Bagri A, Mattevi C, Acik M, Chabal YJ, Chhowalla M, Shenoy VB. 2010. Structural evolution during the reduction of chemically derived graphene oxide. Nature Chem. 2(7):581-587. https://doi.org/10.1038/nchem.686
11.Mattevi C, Eda G, Agnoli S, Miller S, Mkhoyan KA, Celik O, Mastrogiovanni D, Granozzi G, Garfunkel E, Chhowalla M. 2009. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Funct. Mater.. 19(16):2577-2583. https://doi.org/10.1002/adfm.200900166
12.Park S, Ruoff RS. 2009. Chemical methods for the production of graphenes. Nature Nanotech. 4(4):217-224. https://doi.org/10.1038/nnano.2009.58
13.Panzavolta S, Bracci B, Gualandi C, Focarete ML, Treossi E, Kouroupis-Agalou K, Rubini K, Bosia F, Brely L, Pugno NM, et al. 2014. Structural reinforcement and failure analysis in composite nanofibers of graphene oxide and gelatin. Carbon. 78566-577. https://doi.org/10.1016/j.carbon.2014.07.040
14.Zhao X, Xu Z, Zheng B, Gao C. 2013. Macroscopic assembled, ultrastrong and H2SO4-resistant fibres of polymer-grafted graphene oxide. Sci Rep. 3(1): https://doi.org/10.1038/srep03164
15.Schopp S, Thomann R, Ratzsch K, Kerling S, Altstädt V, Mülhaupt R. 2014. Functionalized Graphene and Carbon Materials as Components of Styrene-Butadiene Rubber Nanocomposites Prepared by Aqueous Dispersion Blending. Macromol. Mater. Eng.. 299(3):319-329. https://doi.org/10.1002/mame.201300127
16.Borini S, White R, Wei D, Astley M, Haque S, Spigone E, Harris N, Kivioja J, Ryhänen T. 2013. Ultrafast Graphene Oxide Humidity Sensors. ACS Nano. 7(12):11166-11173. https://doi.org/10.1021/nn404889b
17.Prezioso S, Perrozzi F, Giancaterini L, Cantalini C, Treossi E, Palermo V, Nardone M, Santucci S, Ottaviano L. 2013. Graphene Oxide as a Practical Solution to High Sensitivity Gas Sensing. J. Phys. Chem. C. 117(20):10683-10690. https://doi.org/10.1021/jp3085759
18.Liscio A, Veronese GP, Treossi E, Suriano F, Rossella F, Bellani V, Rizzoli R, Samorì P, Palermo V. 2011. Charge transport in graphene?polythiophene blends as studied by Kelvin Probe Force Microscopy and transistor characterization. J. Mater. Chem. 21(9):2924. https://doi.org/10.1039/c0jm02940h
19.Rapino S, Treossi E, Palermo V, Marcaccio M, Paolucci F, Zerbetto F. Playing peekaboo with graphene oxide: a scanning electrochemical microscopy investigation. Chem. Commun.. 50(86):13117-13120. https://doi.org/10.1039/c4cc06368f
20.Liu Z, Parvez K, Li R, Dong R, Feng X, Müllen K. 2015. Transparent Conductive Electrodes from Graphene/PEDOT:PSS Hybrid Inks for Ultrathin Organic Photodetectors. Adv. Mater. 27(4):669-675. https://doi.org/10.1002/adma.201403826
21.Palermo V, Samorì P. 2007. Molecular Self-Assembly across Multiple Length Scales. Angew. Chem. Int. Ed. 46(24):4428-4432. https://doi.org/10.1002/anie.200700416
22.Xia ZY, Pezzini S, Treossi E, Giambastiani G, Corticelli F, Morandi V, Zanelli A, Bellani V, Palermo V. 2013. Graphene: The Exfoliation of Graphene in Liquids by Electrochemical, Chemical, and Sonication-Assisted Techniques: A Nanoscale Study (Adv. Funct. Mater. 37/2013). Adv. Funct. Mater. 23(37):4756-4756. https://doi.org/10.1002/adfm.201370188
23.Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS. 2012. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 112(11):6156-6214. https://doi.org/10.1021/cr3000412
24.Englert JM, Röhrl J, Schmidt CD, Graupner R, Hundhausen M, Hauke F, Hirsch A. 2009. Soluble Graphene: Generation of Aqueous Graphene Solutions Aided by a Perylenebisimide-Based Bolaamphiphile. Adv. Mater. 21(42):4265-4269. https://doi.org/10.1002/adma.200901578
25.Backes C, Schmidt CD, Rosenlehner K, Hauke F, Coleman JN, Hirsch A. 2010. Nanotube Surfactant Design: The Versatility of Water-Soluble Perylene Bisimides. Adv. Mater.. 22(7):788-802. https://doi.org/10.1002/adma.200902525
26.Yang H, Hernandez Y, Schlierf A, Felten A, Eckmann A, Johal S, Louette P, Pireaux J, Feng X, Mullen K, et al. 2013. A simple method for graphene production based on exfoliation of graphite in water using 1-pyrenesulfonic acid sodium salt. Carbon. 53357-365. https://doi.org/10.1016/j.carbon.2012.11.022
27.Parviz D, Das S, Ahmed HST, Irin F, Bhattacharia S, Green MJ. 2012. Dispersions of Non-Covalently Functionalized Graphene with Minimal Stabilizer. ACS Nano. 6(10):8857-8867. https://doi.org/10.1021/nn302784m
28.Su Q, Pang S, Alijani V, Li C, Feng X, Müllen K. 2009. Composites of Graphene with Large Aromatic Molecules. Adv. Mater.. 21(31):3191-3195. https://doi.org/10.1002/adma.200803808
29.An X, Butler TW, Washington M, Nayak SK, Kar S. 2011. Optical and Sensing Properties of 1-Pyrenecarboxylic Acid-Functionalized Graphene Films Laminated on Polydimethylsiloxane Membranes. ACS Nano. 5(2):1003-1011. https://doi.org/10.1021/nn102415c
30.Geng J, Kong B, Yang SB, Jung H. 2010. Preparation of graphene relying on porphyrin exfoliation of graphite. Chem. Commun.. 46(28):5091. https://doi.org/10.1039/c001609h
31.Xu Y, Zhao L, Bai H, Hong W, Li C, Shi G. 2009. Chemically Converted Graphene Induced Molecular Flattening of 5,10,15,20-Tetrakis(1-methyl-4-pyridinio) porphyrin and Its Application for Optical Detection of Cadmium (II) Ions. J. Am. Chem. Soc.. 131(37):13490-13497. https://doi.org/10.1021/ja905032g
32.Xu Y, Liu Z, Zhang X, Wang Y, Tian J, Huang Y, Ma Y, Zhang X, Chen Y. 2009. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater.. 21(12):1275-1279. https://doi.org/10.1002/adma.200801617
33.Tu W, Lei J, Zhang S, Ju H. 2010. Characterization, Direct Electrochemistry, and Amperometric Biosensing of Graphene by Noncovalent Functionalization with Picket-Fence Porphyrin. Chem. Eur. J.. 16(35):10771-10777. https://doi.org/10.1002/chem.201000620
34.Geng J, Jung H. 2010. Porphyrin Functionalized Graphene Sheets in Aqueous Suspensions: From the Preparation of Graphene Sheets to Highly Conductive Graphene Films. J. Phys. Chem. C. 114(18):8227-8234. https://doi.org/10.1021/jp1008779
35.Zhang X, Huang Y, Wang Y, Ma Y, Liu Z, Chen Y. 2009. Synthesis and characterization of a graphene? C60 hybrid material. Carbon. 47(1):334-337. https://doi.org/10.1016/j.carbon.2008.10.018
36.Melucci M, Treossi E, Ortolani L, Giambastiani G, Morandi V, Klar P, Casiraghi C, Samorì P, Palermo V. 2010. Facile covalent functionalization of graphene oxide using microwaves: bottom-up development of functional graphitic materials. J. Mater. Chem.. 20(41):9052. https://doi.org/10.1039/c0jm01242d
37.Melucci M, Durso M, Zambianchi M, Treossi E, Xia Z, Manet I, Giambastiani G, Ortolani L, Morandi V, De Angelis F, et al. 2012. Graphene?organic hybrids as processable, tunable platforms for pH-dependent photoemission, obtained by a new modular approach. J. Mater. Chem.. 22(35):18237. https://doi.org/10.1039/c2jm33349j
38.Liu Y, Zhou J, Zhang X, Liu Z, Wan X, Tian J, Wang T, Chen Y. 2009. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon. 47(13):3113-3121. https://doi.org/10.1016/j.carbon.2009.07.027
39.Saxena AP, Deepa M, Joshi AG, Bhandari S, Srivastava AK. 2011. Poly(3,4-ethylenedioxythiophene)-Ionic Liquid Functionalized Graphene/Reduced Graphene Oxide Nanostructures: Improved Conduction and Electrochromism. ACS Appl. Mater. Interfaces. 3(4):1115-1126. https://doi.org/10.1021/am101255a
40.Quintana M, Montellano A, del Rio Castillo AE, Tendeloo GV, Bittencourt C, Prato M. 2011. Selective organic functionalization of graphene bulk or graphene edges. Chem. Commun.. 47(33):9330. https://doi.org/10.1039/c1cc13254g
41.Su C, Tandiana R, Balapanuru J, Tang W, Pareek K, Nai CT, Hayashi T, Loh KP. 2015. Tandem Catalysis of Amines Using Porous Graphene Oxide. J. Am. Chem. Soc.. 137(2):685-690. https://doi.org/10.1021/ja512470t
42.He S, Song B, Li D, Zhu C, Qi W, Wen Y, Wang L, Song S, Fang H, Fan C. 2010. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater.. 20(3):453-459. https://doi.org/10.1002/adfm.200901639
43.Patil AJ, Vickery JL, Scott TB, Mann S. 2009. Aqueous Stabilization and Self-Assembly of Graphene Sheets into Layered Bio-Nanocomposites using DNA. Adv. Mater.. 21(31):3159-3164. https://doi.org/10.1002/adma.200803633
44.Yang H, Withers F, Gebremedhn E, Lewis E, Britnell L, Felten A, Palermo V, Haigh S, Beljonne D, Casiraghi C. Dielectric nanosheets made by liquid-phase exfoliation in water and their use in graphene-based electronics. 2D Mater.. 1(1):011012. https://doi.org/10.1088/2053-1583/1/1/011012
45.Schlierf A, Cha K, Georg Schwab M, Samor? P, Palermo V. Exfoliation of graphene with an industrial dye: teaching an old dog new tricks. 2D Mater.. 1(3):035006. https://doi.org/10.1088/2053-1583/1/3/035006
46.Sinitskii A, Dimiev A, Corley DA, Fursina AA, Kosynkin DV, Tour JM. 2010. Kinetics of Diazonium Functionalization of Chemically Converted Graphene Nanoribbons. ACS Nano. 4(4):1949-1954. https://doi.org/10.1021/nn901899j
47.Niyogi S, Bekyarova E, Itkis ME, Zhang H, Shepperd K, Hicks J, Sprinkle M, Berger C, Lau CN, deHeer WA, et al. 2010. Spectroscopy of Covalently Functionalized Graphene. Nano Lett.. 10(10):4061-4066. https://doi.org/10.1021/nl1021128
48.Coletti C, Riedl C, Lee DS, Krauss B, Patthey L, von Klitzing K, Smet JH, Starke U. Charge neutrality and band-gap tuning of epitaxial graphene on SiC by molecular doping. Phys. Rev. B. 81(23): https://doi.org/10.1103/physrevb.81.235401
49.Zhang Y, Zhou K, Xie K, Zeng J, Zhang H, Peng Y. 2010. Tuning the electronic structure and transport properties of graphene by noncovalent functionalization: effects of organic donor, acceptor and metal atoms. Nanotechnology. 21(6):065201. https://doi.org/10.1088/0957-4484/21/6/065201
50.Dong X, Fu D, Fang W, Shi Y, Chen P, Li L. 2009. Doping Single-Layer Graphene with Aromatic Molecules. Small. 5(12):1422-1426. https://doi.org/10.1002/smll.200801711
51.Melucci M, Treossi E, Ortolani L, Giambastiani G, Morandi V, Klar P, Casiraghi C, Samorì P, Palermo V. 2010. Facile covalent functionalization of graphene oxide using microwaves: bottom-up development of functional graphitic materials. J. Mater. Chem.. 20(41):9052. https://doi.org/10.1039/c0jm01242d
52.Treossi E, Melucci M, Liscio A, Gazzano M, Samori? P, Palermo V. 2009. High-Contrast Visualization of Graphene Oxide on Dye-Sensitized Glass, Quartz, and Silicon by Fluorescence Quenching. J. Am. Chem. Soc.. 131(43):15576-15577. https://doi.org/10.1021/ja9055382
53.Kim J, Cote LJ, Kim F, Huang J. 2010. Visualizing Graphene Based Sheets by Fluorescence Quenching Microscopy. J. Am. Chem. Soc.. 132(1):260-267. https://doi.org/10.1021/ja906730d
54.Gaudreau L, Tielrooij KJ, Prawiroatmodjo GEDK, Osmond J, de Abajo FJG, Koppens FHL. 2013. Universal Distance-Scaling of Nonradiative Energy Transfer to Graphene. Nano Lett.. 13(5):2030-2035. https://doi.org/10.1021/nl400176b
55.Ciesielski A, Haar S, El? Gemayel M, Yang H, Clough J, Melinte G, Gobbi M, Orgiu E, Nardi MV, Ligorio G, et al. 2014. Harnessing the Liquid-Phase Exfoliation of Graphene Using Aliphatic Compounds: A Supramolecular Approach. Angew. Chem. Int. Ed.. 53(39):10355-10361. https://doi.org/10.1002/anie.201402696
56.El Gemayel M, Haar S, Liscio F, Schlierf A, Melinte G, Milita S, Ersen O, Ciesielski A, Palermo V, Samorì P. 2014. Leveraging the Ambipolar Transport in Polymeric Field-Effect Transistors via Blending with Liquid-Phase Exfoliated Graphene. Adv. Mater.. 26(28):4814-4819. https://doi.org/10.1002/adma.201400895
57.Mativetsky JM, Liscio A, Treossi E, Orgiu E, Zanelli A, Samori? P, Palermo V. 2011. Graphene Transistors via in Situ Voltage-Induced Reduction of Graphene-Oxide under Ambient Conditions. J. Am. Chem. Soc.. 133(36):14320-14326. https://doi.org/10.1021/ja202371h
58.Mativetsky JM, Treossi E, Orgiu E, Melucci M, Veronese GP, Samori? P, Palermo V. 2010. Local Current Mapping and Patterning of Reduced Graphene Oxide. J. Am. Chem. Soc.. 132(40):14130-14136. https://doi.org/10.1021/ja104567f
59.Liu Y, Yuan L, Yang M, Zheng Y, Li L, Gao L, Nerngchamnong N, Nai CT, Sangeeth CSS, Feng YP, et al. 2014. Giant enhancement in vertical conductivity of stacked CVD graphene sheets by self-assembled molecular layers. Nat Commun. 5(1): https://doi.org/10.1038/ncomms6461
60.Tsoufis T, Tuci G, Caporali S, Gournis D, Giambastiani G. 2013. p-Xylylenediamine intercalation of graphene oxide for the production of stitched nanostructures with a tailored interlayer spacing. Carbon. 59100-108. https://doi.org/10.1016/j.carbon.2013.02.059
61.Ji L, Tan Z, Kuykendall T, An EJ, Fu Y, Battaglia V, Zhang Y. 2011. Multilayer nanoassembly of Sn-nanopillar arrays sandwiched between graphene layers for high-capacity lithium storage. Energy Environ. Sci.. 4(9):3611. https://doi.org/10.1039/c1ee01592c
62.Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng H. 2011. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nature Mater. 10(6):424-428. https://doi.org/10.1038/nmat3001
63.Xia ZY, Wei D, Anitowska E, Bellani V, Ortolani L, Morandi V, Gazzano M, Zanelli A, Borini S, Palermo V. 2015. Electrochemically exfoliated graphene oxide/iron oxide composite foams for lithium storage, produced by simultaneous graphene reduction and Fe(OH)3 condensation. Carbon. 84254-262. https://doi.org/10.1016/j.carbon.2014.12.007
64.Yao H, Ge J, Wang C, Wang X, Hu W, Zheng Z, Ni Y, Yu S. 2013. A Flexible and Highly Pressure-Sensitive Graphene-Polyurethane Sponge Based on Fractured Microstructure Design. Adv. Mater.. 25(46):6692-6698. https://doi.org/10.1002/adma.201303041
65.Mecklenburg M, Schuchardt A, Mishra YK, Kaps S, Adelung R, Lotnyk A, Kienle L, Schulte K. 2012. Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance. Adv. Mater.. 24(26):3486-3490. https://doi.org/10.1002/adma.201200491
66.Zou J, Kim F. 2014. Diffusion driven layer-by-layer assembly of graphene oxide nanosheets into porous three-dimensional macrostructures. Nat Commun. 5(1): https://doi.org/10.1038/ncomms6254
67.Barg S, Perez FM, Ni N, do Vale Pereira P, Maher RC, Garcia-Tuñon E, Eslava S, Agnoli S, Mattevi C, Saiz E. 2014. Mesoscale assembly of chemically modified graphene into complex cellular networks. Nat Commun. 5(1): https://doi.org/10.1038/ncomms5328
68.Wei W, Yang S, Zhou H, Lieberwirth I, Feng X, Müllen K. 2013. 3D Graphene Foams Cross-linked with Pre-encapsulated Fe3O4Nanospheres for Enhanced Lithium Storage. Adv. Mater.. 25(21):2909-2914. https://doi.org/10.1002/adma.201300445
69.Wu Z, Yang S, Sun Y, Parvez K, Feng X, Müllen K. 2012. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc.. 134(22):9082-9085. https://doi.org/10.1021/ja3030565
70.Jin Z, Lu A, Xu Y, Zhang J, Li W. 2014. Ionic Liquid-Assisted Synthesis of Microporous Carbon Nanosheets for Use in High Rate and Long Cycle Life Supercapacitors. Adv. Mater.. 26(22):3700-3705. https://doi.org/10.1002/adma.201306273
71.Luo B, Wang B, Liang M, Ning J, Li X, Zhi L. 2012. Reduced Graphene Oxide-Mediated Growth of Uniform Tin-Core/Carbon-Sheath Coaxial Nanocables with Enhanced Lithium Ion Storage Properties. Adv. Mater.. 24(11):1405-1409. https://doi.org/10.1002/adma.201104362
72.Han S, Wu D, Li S, Zhang F, Feng X. 2014. Porous Graphene Materials for Advanced Electrochemical Energy Storage and Conversion Devices. Adv. Mater.. 26(6):849-864. https://doi.org/10.1002/adma.201303115