Research progress of introducing difluoromethyl
Introduction
The introduction of fluorine atoms into bioactive organic compounds has become one of the important strategies for drug design and lead compound optimization. In drug synthesis strategies, fluoride is often used to change biological related properties, such as metabolic stability, alkalinity, ability to form hydrogen bonds (HB), lipophilicity and bioavailability. In addition, the binding affinity between compounds and biological targets may also be improved after fluorination. Therefore, fluorine is considered as an excellent candidate for the bioelectronic excretion of hydroxyl, thiol or amine groups.

Fig 1 Difluoromethyl group
Most of the fluoride compounds in the marketed drugs contain a single fluorine atom or trifluoromethyl. Due to some unique advantages of difluoromethyl due to its special chemical and physical properties, people are increasingly interested in organic compounds containing difluoromethyl. As a lipophilic hydrogen bond donor, difluoromethyl is similar to thiophenol, aniline and amino group in scale, but different from hydroxyl group. In particular, compared with the situation that the lipophilicity changes dramatically when trifluoromethyl is substituted for methyl, when the trifluoromethyl is substituted, its lipophilicity is only moderately increased, and it can also have the property of hydrogen bond donor. Examples show that the trifluoromethyl can form a hydrogen bond with the target, which can strengthen the interaction between drug molecules and the target 1. For example, a series of ArOCF2H compounds exhibit high lipophilicity and hydrogen bond acidity (Fig. 2).
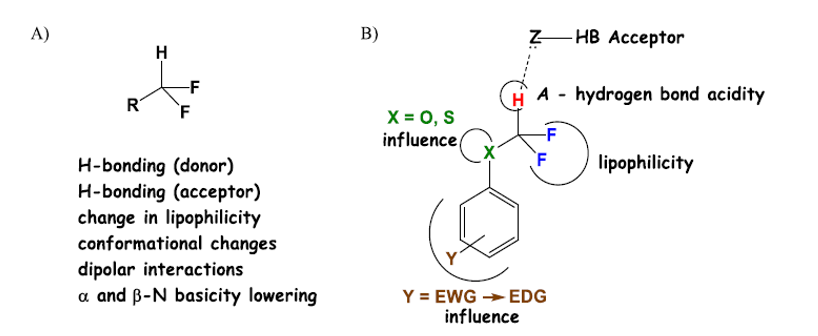
Fig 2 Physicochemical properties of CF2H group
Difluoromethylation
1. Fluorinating reagent
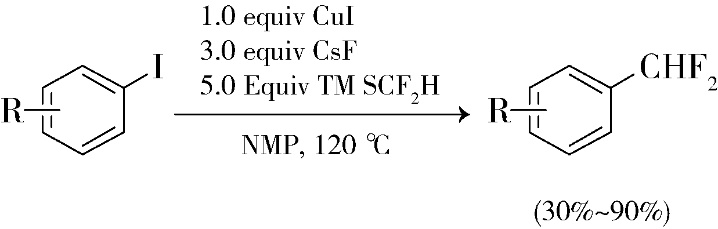
Hartwig research group prepared a new difluoromethylation reagent TMSCF2H by reducing the famous Rupert Prakash regant (TMSCF3) with sodium borohydride 2. This reagent can realize the copper salt catalyzed difluoromethylation of iodobenzene, with high yield and simple operation (Scheme 1). However, this reaction is only applicable to electron rich iodobenzene substrate, and a large amount of difluoromethylation reagent (5 equivalent) needs to be added to the reaction. In addition, the reaction cannot be compatible with functional groups such as aldehyde and ketone, otherwise the carbonyl group will undergo nucleophilic addition.
Scheme 1
On the other hand, Prakash research group completed the difluoromethylation of iodobenzene substrate with tributyl difluoromethyl tin reagent n-Bu3SnCF2H under the catalysis of copper salt, and the reaction temperature was 100 to 120 ° C 3 (Scheme 2). Different from TMSCF2H, the difluoromethyl tin reagent can react with the substrate containing electron withdrawing group, and is compatible with functional groups such as aldehyde and ketone.
Scheme 2
2.Fluorinated building blocks
It is one of the important methods to synthesize fluorine-containing organic compounds by using fluorine-containing intermediates as synthetic blocks and then synthesizing fluorine-containing target compounds through appropriate reaction routes. On the one hand, the use of fluoride blocks is conducive to the industrial synthesis of target molecules; On the other hand, compared with fluorinated agent, the reaction of fluorinated building blocks does not involve the breaking and formation of C-F bond, but only involves the conversion of ordinary functional groups and the formation of C-C bond, so it has the advantages of mild reaction, good selectivity and high yield. In the synthesis of difluoromethyl containing compounds, the blocks of difluoroacetate and difluoroacetic acid derivatives have received more and more attention because they have functional groups such as carbonyl and alkenyl groups that can be used as reaction sites, which can enable the introduction of difluoromethyl groups into the compounds efficiently, and are more suitable for subsequent industrial production.
3 - (difluoromethyl) - 1-methyl-1H-pyrazole-4-carboxylic acid, a key intermediate of difluoromethylpyrazolamide, can be synthesized with fluorinated building blocks ethyl difluoroacetate as raw material. Under alkaline conditions, the fluorinated
building blocks ethyl difluoroacetate undergoes Clementson condensation reaction, and then conducts condensation elimination reaction with ethyl orthoformate. Next, it cyclizes with methyl hydrazine under low temperature and hydrolyzes to obtain the product 4 (Scheme 3).
Scheme 3
The butene ketone block containing difluoromethyl is used to react with amidines under the condition of sodium alcohol. After the reaction, water is added, cooled, filtered and dried to obtain the corresponding pyrimidine compound containing difluoromethyl 5(Scheme 4).
Scheme 4
Applicaition
Difluoromethyl has extraordinary chemical and physical properties and can be used in drug design. According to the changes required in the molecule, more precise design rules can also be set to replace XCH3 with XCF2H. In addition, the role of difluoromethyl in drug design is closely related to its connected functional group, which is not only a hydrogen bond donor functional group, but also regulates the lipophilicity of molecules. In addition to monofluoro atoms and trifluoromethyl, this provides additional options for introducing fluoro groups into drug structures.
Reference
[1]Zafrani Yossi,Yeffet Dina,Sod-Moriah Gali,Berliner Anat,Amir Dafna,Marciano Daniele,Gershonov Eytan,Saphier Sigal. Difluoromethyl Bioisostere: Examining the "Lipophilic Hydrogen Bond Donor" Concept.[J]. Journal of medicinal chemistry,2017,60(2). https://doi.org/10.1021/acs.jmedchem.6b01691
[2]Fier P S, Hartwig J F. Copper-mediated difluoromethylation of aryl and vinyl iodides[J]. J. Am. Chem. Soc., 2012(134): 5524.
[3]Prakash G K S, Ganesh S K, Jones J, et al. Copper-mediated difluoromethylation of (hetero)aryl iodides and beta-styryl halides with tributyl(difluoromethyl)stannane[J]. Angew. Chem., Int. Ed., 2012(51): 12090.
[4]Stierli D, Walter H, Rajan R, et al. Novel microbiocides:WO, 2009127726[P]. 2009-10-22.
[5]Daniel R, Fandrick D R, Desrosiers J, et al. General and rapid pyrimidine condensation by addressing the rate limiting aromatization[M]. Org. Lett., 2014, 16: 2834-2837.
Aladdin:https://www.aladdinsci.com
List of related products