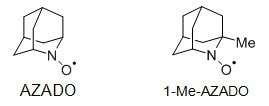TEMPO Catalyzed Oxidations
THE REACTION

TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives play an important role as stabilizing nitroxide radicals for catalysts in organic oxidation reactions. TEMPO was first discovered by Lebedev and Kazarnovskii in 1960. The radical nature of the stabilizing radicals of TEMPO is attributed to the fact that the presence of bulky substituents on its own can prevent the radicals from reacting with other molecules. 1
![]()
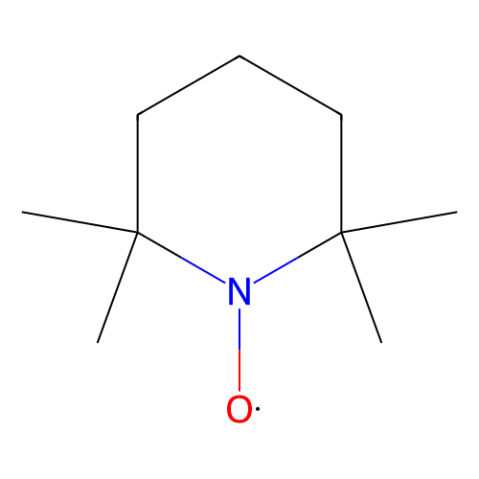
Fig 1. TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl)
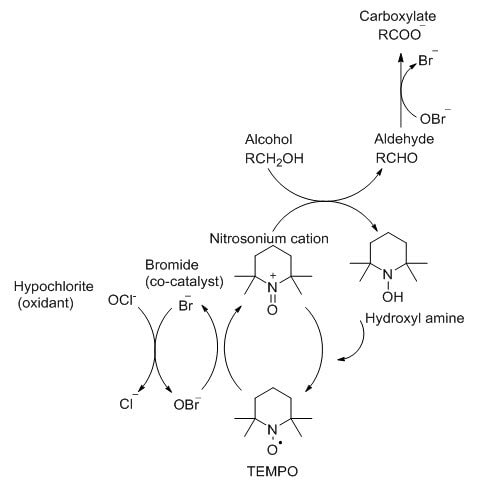
TEMPO and its derivatives are mainly applied to the oxidation of primary and secondary alcohols. TEMPO has good solubility not only in organic solvents but also in aqueous media.[1] In addition, TEMPO can be used in combination with various reoxidizing agents for oxidation in organic media (e.g. hypochlorite) and Cu/O2 for oxidation in organic media.[1] Within aqueous media, TEMPO can be oxidized by an oxidizing agent of the same stoichiometry (sodium hypochlorite) and produces the nitrosonium cation, which serves as the actual oxidizing agent in the oxidation of alcohols. During the oxidation of alcohols, the nitrosonium cation is reduced to hydroxylamine, which is then oxidized back to the nitrosonium cation by a suitable oxidant to complete the catalytic cycle. Hypochlorite is used as the primary oxidizing agent and bromide is used as a co-catalyst. [2]
![]()
Fig 2.Mechanism of the TEMPO mediated oxidation of alcohols[2]
TEMPO (2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are also used as catalysts in other oxidation reactions. They are cost-effective alternatives to heavy metal reagents for highly selective oxidation catalysts and can catalyze reactions involving the formation of C-C, C-O, C=O, C-N, and C=N bonds. [3]
Applications
TEMPO-mediated oxidation reactions have been applied in the following areas:
1,4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl radical (4-amino-TEMPO) -mediated oxidation reaction can be used for the preparation of cellouronic acid and can be analyzed by size-exclusion chromatography coupled with a multi-angle laser scattering detector (SEC-MALS). [4]
2,Compared to TEMPO, 2-Azaadamantane N-oxyl (AZADO) and 1-Me-AZADO showed excellent catalytic ability for applications that can convert various sterically hindered alcohols into the corresponding carbonyl compounds. [5]
Fig 3. 2-Azadamantane N-yloxy (AZADO) and 1-Methyl-2-azadamantane-N-yloxy (1-Me-AZAADO)
1,TEMPO can be used to catalyze the oxidation of regenerated cellulose (viscose rayon).TEMPO oxidizes the C6 primary hydroxyl group in rayon to a carboxyl group, making the product water soluble. 6
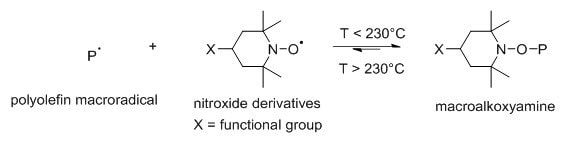
2,TEMPO derivatives are now used in post-polymerization modification processes due to their ability to graft polar substances (e.g., hydroxyl or ester groups) onto polymers. 4-Hydroxy-TEMPO (HO-TEMPO) and 4-Benzoyloxy-TEMPO (BzO-TEMPO) can be applied as functionalization reagents for the poly[ethylene-co-(1-octene)] efficient functionalization. It can graft polar functional groups by rapid coupling of nitroxide radicals with carbon-centered radicals such as macroradicals. 7
Fig 4. Nitroxide derivatives-and macroradical
1,Coupling reactions between nitroxide derivatives and macroradical. 2
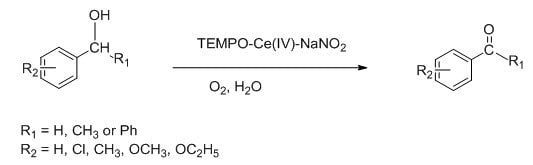
2,The catalytic system of lTEMPO, Ce(IV) and NaNO2 has now been applied to the selective oxidation reaction of different alcohols. The corresponding aldehydes and ketones generated after oxidation of alcohols in this reaction were produced in 45.5-98.0% yields. 8
Fig 5.TEMPO-Ce(IV)-NANO2
1,The TEMPO-catalyzed synthesis of xanthan can be carried out by regioselective oxidation to synthesize the xanthouronic acid sodium salt (xanthouronan). The reaction process through 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and hydroxyl radicals can be used to evaluate the antioxidant activity of xanthan. 9
2,The preparation of pure (1→3)-β-polyglucuronic acid sodium salt has been reported at by 4-acetamido-TEMPO/NaClO/NaClO2-catalyzed oxidation of curdlan. 10
3,The optimization of this reaction for the preparation of nanofibrous cellulose (NFC) from date palm was achieved by monitoring the degree of oxidation of pristine cellulose catalyzed by TEMPO. 11
RECENT RESEARCH AND TRENDS
1,Stabilized nitroxide radical organic polymer materials have been developed that have important applications in the field of energy storage materials. [12]
2,ThelCu/TEMPO catalyst system offers high efficiency and selectivity in the aerobic oxidative lactonization reaction of diols. [13]
3,Surface-active aldehyde groups can be introduced by chemo-enzymatic modification of cellulose nanofibers (CNFs) under the catalytic conditions of laccase (biocatalyst) and TEMPO or 4-amino-TEMPO (mediators). [14]
4,Electrolytic conversion of diphenylmethanol to benzophenone in the presence of solid-solid 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO). [15]
5,Eco-friendly electrochemical oxidation of alcohols by replacing expensive transition metal catalysts with TEMPO as an electroactive organocatalyst. This electrocatalytic system has been used as a fast, simple, selective and waste-free oxidation scheme for various alcohols. [16]
6,Magnetically separable organocatalyst (Fe3O4@SiO2-TEMPO) under metal and halogen free conditions. Aerobic oxidation of 5-hydroxymethylfurfural (5-HMF) to 2,5-diformylfuran (DFF) was possible and the reaction was highly selective. [17]
7,A light-responsive delivery system consisting of TEMPO oxidized Konjac glucomannan (OKGM) polymer has been prepared. [18]
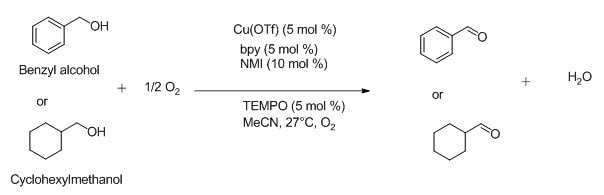
8,(bpy)CuI/TEMPO/NMI catalyst system (bpy=2,2′-bipyridine, TEMPO=2,2,6,6-tetramethyl-tetramethyl-piperidinyl-N-yloxy, and NMI=N-methylimidazole) has been applied in the rapid and highly selective oxidation of benzyl and aliphatic alcohols. The "(bpy)CuI/TEMPO" catalyst system used in this reaction consisted of MeCN solvent and 5 mol% [Cu(MeCN)4](OTf), 5 mol% bpy, 5 mol% TEMPO and 10 mol% NMI. Among them benzyl alcohol is oxidized faster than fatty alcohol (cyclohexanol). [19]
The scheme of the above oxidation is shown below (Figure 6):
Fig 6. Oxidation
Reference
1.Fernandez MJF, Sato H. 2011. Solvent effect on (2,2,6,6-Tetramethylpiperidine-1-yl)oxyl (TEMPO): a RISM-SCF-SEDD study. Theor Chem Acc. 130(2-3):299-304. https://doi.org/10.1007/s00214-011-0976-y
2.Kiricsi I, Nagy J, Karge H, Palyi G. 1999. Porous Materials in Environmentally Friendly Processes. Proceedings of the 1st International FEZA Conference; 03 Sep 1999; Eger (Hungary)
3.Zhou Z, Liu L. 2014. TEMPO and its Derivatives: Synthesis and Applications. COC. 18(4):459-474. https://doi.org/10.2174/13852728113176660151
4.Shibata I, Yanagisawa M, Saito T, Isogai A. 2006. SEC-MALS analysis of cellouronic acid prepared from regenerated cellulose by TEMPO-mediated oxidation. Cellulose. 13(1):73-80. https://doi.org/10.1007/s10570-005-9021-4
5.Shibuya M, Tomizawa M, Suzuki I, Iwabuchi Y. 2006. 2-AzaadamantaneN-Oxyl (AZADO) and 1-Me-AZADO: Highly Efficient Organocatalysts for Oxidation of Alcohols. J. Am. Chem. Soc.. 128(26):8412-8413. https://doi.org/10.1021/ja0620336
6.Shibata I, Isogai A. 2003. 10(4):335-341. https://doi.org/10.1023/a:1027330409470
7.Passaglia E, Coiai S, Cicogna F, Ciardelli F. 2014. Some recent advances in polyolefin functionalization. Polym. Int.. 63(1):12-21. https://doi.org/10.1002/pi.4598
8.Yan Y, Tong X, Wang K, Bai X. 2014. Highly efficient and selective aerobic oxidation of alcohols in aqueous media by TEMPO-containing catalytic systems. Catalysis Communications. 43112-115. https://doi.org/10.1016/j.catcom.2013.09.022
9.Delattre C, Pierre G, Gardarin C, Traikia M, Elboutachfaiti R, Isogai A, Michaud P. 2015. Antioxidant activities of a polyglucuronic acid sodium salt obtained from TEMPO-mediated oxidation of xanthan. Carbohydrate Polymers. 11634-41. https://doi.org/10.1016/j.carbpol.2014.04.054
10.Watanabe E, Tamura N, Saito T, Habu N, Isogai A. 2014. Preparation of completely C6-carboxylated curdlan by catalytic oxidation with 4-acetamido-TEMPO. Carbohydrate Polymers. 10074-79. https://doi.org/10.1016/j.carbpol.2012.11.094
11.Benhamou K, Dufresne A, Magnin A, Mortha G, Kaddami H. 2014. Control of size and viscoelastic properties of nanofibrillated cellulose from palm tree by varying the TEMPO-mediated oxidation time. Carbohydrate Polymers. 9974-83. https://doi.org/10.1016/j.carbpol.2013.08.032
12.Hughes BK, Braunecker WA, Ferguson AJ, Kemper TW, Larsen RE, Gennett T. 2014. Quenching of the Perylene Fluorophore by Stable Nitroxide Radical-Containing Macromolecules. J. Phys. Chem. B. 118(43):12541-12548. https://doi.org/10.1021/jp506240j
13.Xie X, Stahl SS. 2015. Efficient and Selective Cu/Nitroxyl-Catalyzed Methods for Aerobic Oxidative Lactonization of Diols. J. Am. Chem. Soc.. 137(11):3767-3770. https://doi.org/10.1021/jacs.5b01036
14.Jauovec D, Vogrini R, Kokol V. 2015. Introduction of aldehyde vs. carboxylic groups to cellulose nanofibers using laccase/TEMPO mediated oxidation. Carbohydrate Polymers. 11674-85. https://doi.org/10.1016/j.carbpol.2014.03.014
15.Kaluza D, Jönsson-Niedziólka M, Ahn SD, Owen RE, Jones MD, Marken F. 2015. Solid-solid EC TEMPO-electrocatalytic conversion of diphenylcarbinol to benzophenone. J Solid State Electrochem. 19(5):1277-1283. https://doi.org/10.1007/s10008-014-2722-6
16.Karimi B, Rafiee M, Alizadeh S, Vali H. Eco-friendly electrocatalytic oxidation of alcohols on a novel electro generated TEMPO-functionalized MCM-41 modified electrode. Green Chem.. 17(2):991-1000. https://doi.org/10.1039/c4gc01303d
17.Karimi B, Mirzaei HM, Farhangi E. 2014. Fe3O4@SiO2-TEMPO as a Magnetically Recyclable Catalyst for Highly Selective Aerobic Oxidation of 5-Hydroxymethylfurfural into 2,5-Diformylfuran under Metal- and Halogen-Free Conditions. ChemCatChem. 6(3):758-762. https://doi.org/10.1002/cctc.201301081
18.Chen X, Wang S, Lu M, Chen Y, Zhao L, Li W, Yuan Q, Norde W, Li Y. 2014. Formation and Characterization of Light-Responsive TEMPO-Oxidized Konjac Glucomannan Microspheres. Biomacromolecules. 15(6):2166-2171. https://doi.org/10.1021/bm500327m
19.Hoover JM, Ryland BL, Stahl SS. 2013. Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc.. 135(6):2357-2367. https://doi.org/10.1021/ja3117203