Acrylonitrile
Product Manager:Nick Wilde
Acrylonitrile is an organic compound with the formula CH2CHCN and the structure H2C=CH−C≡N. It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. It is a kind of conjugated unsaturated nitrile, and the carbon-carbon double bond and cyano group in the molecule can participate in various reactions, such as hydration to acrylamide and acrylic acid, diene synthesis with dienophile, hydrogenation to propiononitrile and propylamine, copolymerization with acrylamide, electrolytic coupling to hexanedinitrile, as well as being used as a cyanohydroethylation reagent for alcohols and amines. It is reactive and toxic at low doses.
Acrylonitrile is one of the components of ABS plastic (Acrylonitrile butadiene styrene).

Application
Acrylonitrile is mainly used as a monomer in the manufacture of polymers, including polyacrylonitrile, acrylic fibers and nitrile rubber. Small amounts of acrylonitrile are also used in fumigants. In addition, acrylonitrile and its derivatives, such as 2-chloroacrylonitrile, are capable of undergoing the Diels-Alder reaction.
Recent Literature
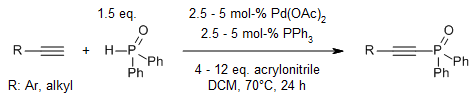
A silver-free palladium-catalyzed dehydrogenative phosphorylation of terminal alkynes with hydrogen phosphine oxides produces the corresponding value-added alkynylphosphine oxides in very good yields. This reaction could be easily conducted at gram scales without any decrease of efficiency.
J.-Q. Zhang, T. Chen, J.-S. Zhang, L.-B. Han, Org. Lett., 2017, 19, 4692-4695. https://doi.org/10.1021/acs.orglett.7b02389
Quoted from:
https://en.wikipedia.org/wiki/Acrylonitrile
https://www.organic-chemistry.org/chemicals/oxidations/acrylonitrile.shtm
Aladdin:https://www.aladdinsci.com
