Antibody-coupled drug ADCs: Precise guidance, cancer destruction

Product Manager:
Harrison Michael
Antibody-coupled drug ADCs are a hot frontier in cancer therapy, providing a targeted therapy approach that combines the specificity of monoclonal antibodies with the potency of cytotoxic agents. The concept of ADC can be traced back to the theory of targeting cells for compound delivery proposed by German Nobel laureate Paul Ehrlich in 1897, and was first applied clinically by Mathe in 1958, who combined methylaminopterin with anti-mouse immunoglobulin for the treatment of leukemia. In November 2022, the U.S. FDA granted accelerated approval to Elahere® (Mirvetuximab soravtansine-gyynx), marking a breakthrough as the first ADC agent for the treatment of platinum-resistant ovarian cancer. Similarly, in February 2022, the China Food and Drug Administration approved HER ADC injection of 8-Aminoguanosine (Enhertu®) (CAS number: 180288-69-1) for the treatment of metastatic breast cancer, suggesting a new therapeutic avenue. So far, ADC drugs have made further progress in improving efficacy and reducing toxicity through development breakthroughs.
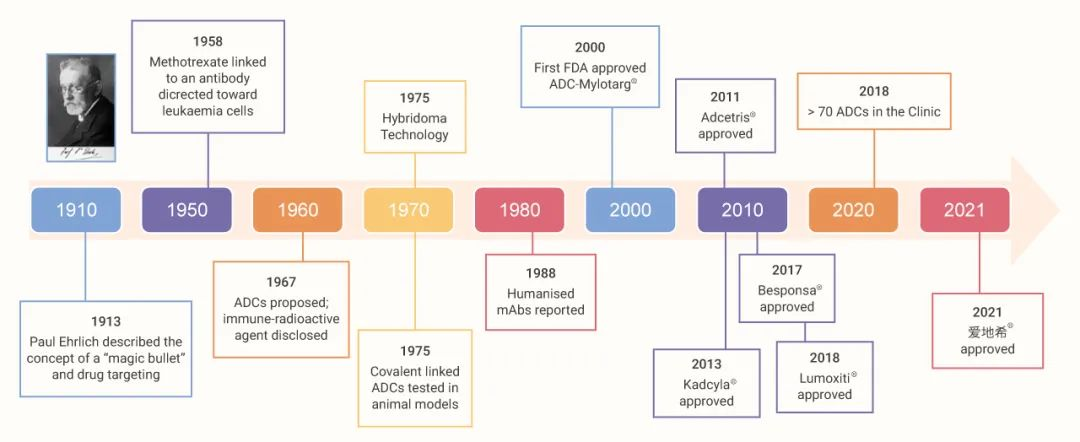
Figure 1:History of the development of ADC drugs¹
Mechanism of action
Antibody-drug conjugates (ADCs) exploit the tumor-targeting precision of monoclonal antibodies to selectively bind tumor antigens. This targeted binding is accomplished by coupling a monoclonal antibody targeting a specific antigen with a small molecule cytotoxic drug through a linker. The unique combination of a monoclonal antibody and a cytotoxic payload enables precise identification of tumor cells and direct delivery of effective anticancer agents to the tumor site.
The mechanism of ADC action includes several key steps. First, the monoclonal antibody component of ADC specifically recognizes and binds to antigens on the surface of tumor cells. This interaction promotes the internalization of ADC-drug complexes into tumor cells through receptor-mediated endocytosis.
Once inside the tumor cell, the linker linking the antibody to the cytotoxic drug undergoes cleavage in response to intracellular conditions such as low pH or enzymatic activity. This division releases cytotoxic drugs, which then exert their anticancer effects by interfering with fundamental cellular processes such as DNA replication or microtubule assembly.
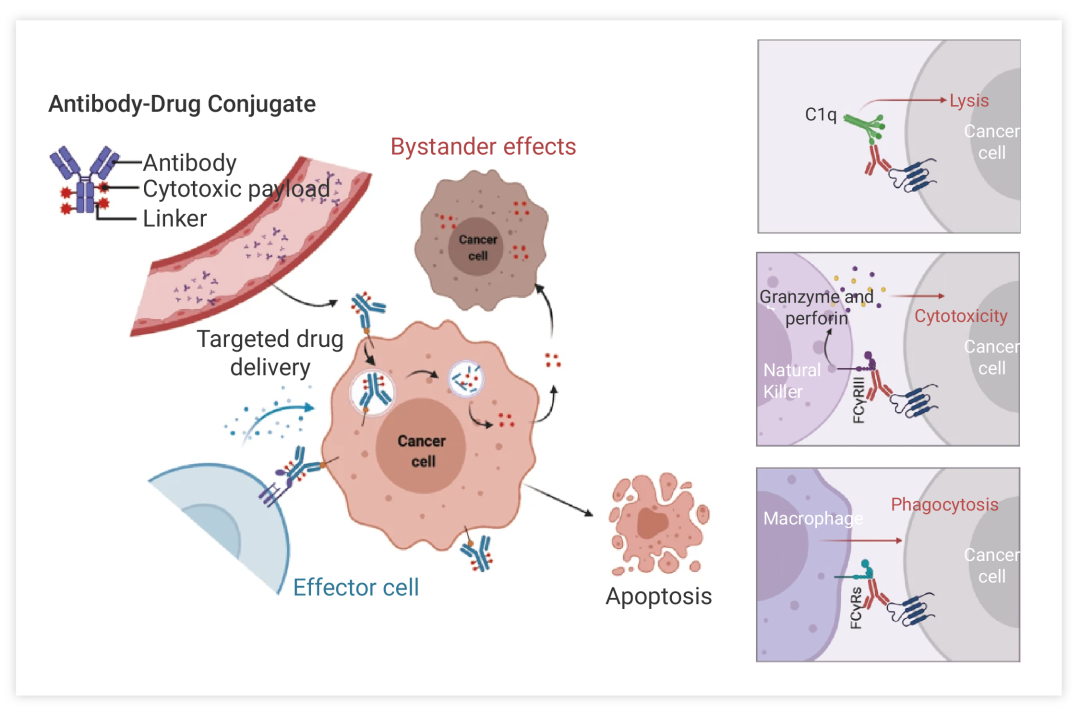
Figure 2:The main core mechanism of action of ADC²
In this mechanism, because ADCs selectively target tumor cells expressing antigens recognized by monoclonal antibodies, they can specifically deliver cytotoxic agents to cancer tissues while protecting healthy cells. This targeting approach minimizes systemic toxicity and reduces the side effects associated with conventional chemotherapy that typically affect rapidly dividing normal cells throughout the body. In other words, it takes advantage of the specificity of monoclonal antibodies and the potency of cytotoxic drugs to deliver targeted therapy directly to tumor cells, with the potential to improve efficacy and reduce toxicity compared with traditional chemotherapy.
Over the years, ADC has undergone three different generations of transformation, each addressing the limitations of the previous generation. The first generation, using Mylotarg® and Besponsa® as examples, introduced mouse antibodies and acid cleavage linker, but faced challenges of off-target effects and immunogenicity. Second-generation ADCs, including Kadcyla®, address these shortcomings with nonlytic linker and extended linker types, despite off-target toxicity and drug resistance issues. The third generation, represented by Enhertu®, further improves fully humanized antibodies, hydrophilic linkers, and site-specific conjugation with lower toxicity and higher efficacy.

Figure 3:ADC associated target antigens in solid tumors³
Challenges and future prospects
Despite the remarkable progress, challenges remain, particularly regarding off-target toxicity and the narrow therapeutic window. However, continuous improvements in antibody engineering, linker design, payload selection, and conjugation techniques are expected to expand the therapeutic window and advance the development of next-generation ADCs.
Worldwide, ADC research and development continues to flourish, and many clinical trials are ongoing. While the ADC market is growing exponentially, with sales expected to exceed $16.4 billion by 2026, domestic momentum is gaining momentum in countries such as China, marked by the emergence of homegrown ADC therapies.
Looking ahead, with continued innovation, the future of ADC seems promising for further improvement of clinical outcomes and expansion of therapeutic applications. As ADCs continue to redefine the cancer treatment paradigm, they remain at the forefront of cancer drug development, bringing new hope to patients worldwide.
Figure 4:The future direction of ADC development⁴
Conclusion
In conclusion, ADC represents a transformative approach to cancer therapy, with a rich history of innovation and a promising future that marks continued progress. Aladdin Bio power ADC drug research, commitment to quality, diverse product portfolio, customization options, expert support and competitive pricing make it the preferred partner for researchers in the ADC field. Aladdin Biosciences provides a comprehensive range of reagents for antibody-drug conjugate (ADC) research.
Reference
1. D. E. Thurston and P. J M Jackson, “Cytotoxic Payloads for Antibody–Drug Conjugates”M. The Royal Society of Chemistry, 2019. https://www.rsc.org/search-results/?q=David+E+Thurston%2C+Paul+J+M+JacksonCytotoxic+Payloads+for+Antibody
2. Z. W. Fu, S. J. Li, S. F. Han, C. Shi and Y. Zhang,“Antibody drug conjugate: the "biological missile" for targeted cancer therapy,” J. Signal Transduct Target Ther. 7(1), 93 (2022). https://doi.org/10.1038/s41392-022-00947-7
3. N. Diamantis and U. Banerji, “Antibody-drug conjugates--an emerging class of cancer treatment,” J. British journal of cancer, 114(4), 362–367 (2016). https://doi.org/10.1038/bjc.2015.435
4. Paolo Tarantino, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022 Mar;72(2):165-182.
5. P. Tarantino, R. C. Pestana, C. Corti, S Modi, A. Bardia, S M Tolaney, J Cortes, J. C. Soria and G. Curigliano. “Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies,”CA: a cancer journal for clinicians, 72(2), 165–182 (2022). https://doi.org/10.3322/caac.21705
Aladdin:https://www.aladdinsci.com/

