Antibody Isotype Controls
What is an Isotype Control?

An isotype control antibody appears like a typical antibody but it does not attach itself to a specific target protein like the primary antibody used in the experiment.
Isotype control antibodies must have equivalent immunoglobulin class and subclass in comparison to the primary antibody. However, it lacks specificity to the protein of interest.
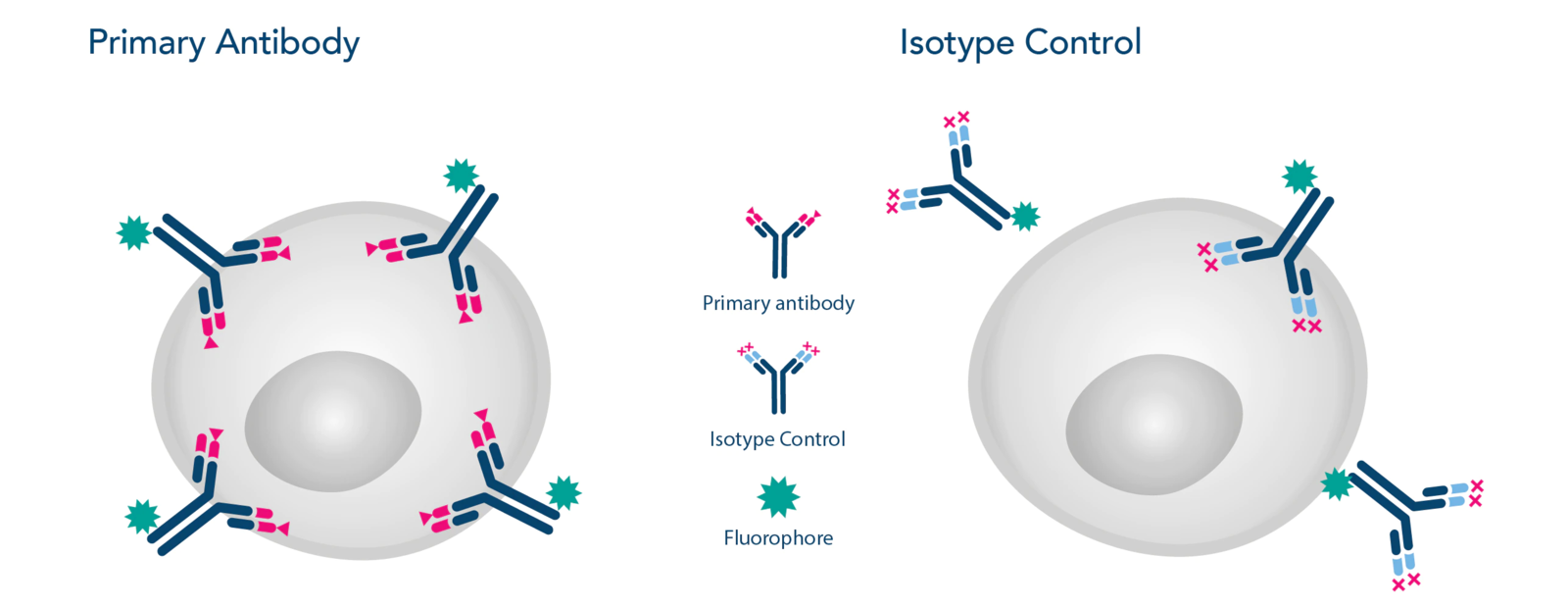
Image 1: Primary Antibody vs. Isotype Control
Why use an Isotype Control?
The term 'isotype control' literally refers to a control. It is an antibody that serves as a negative control in applications related to antibody staining. Its purpose is to help you distinguish between specific antibody staining and background staining in your experiments.
Background staining can occur due to various reasons, including Fc receptor interactions. Even though the Fab region, where the useful target-specific binding occurs, usually gets more attention, the Fc region can also bind. Antibodies and their conjugates may bind non-specifically to other proteins, endogenous enzymes, lipids, and Fc receptors. This is due to the presence of Fc receptors on a variety of cells, proteins, and lipids that are not of interest.
Isotype controls are a specific negative control type that share the same Fc region as the antibody used in the actual experiment, but have a variable region that should not bind with the antigen in the experiment. The Fc region can specifically interact with Fc receptors or complement. Therefore, it is important to control for this "background" when staining cell-surface markers. Moreover, these interactions may occur across species, as rat IgG may interact with mouse Fc receptors. Traditionally, performing flow cytometry requires a diverse panel of isotype controls.
Image 2: Scheme of an antibody with Fc Domain
Advantages of using an Isotype Control
By conducting a control experiment alongside your main study using the isotype control instead of your typical primary antibody, and ensuring all other conditions remain the same (e.g. concentration, buffers, temperature), you can assess the degree of non-specific background and establish a threshold for unstained samples.
The incorporation of an isotype control as a negative control in your inquiry enables you to evaluate whether the blocking and/or washing steps in your procedure are satisfactory.
To demonstrate the specificity of identifying a particular band on a Western Blot or staining for a cell-surface marker in flow cytometry utilizing an antibody, a negative control may be implemented that should not produce comparable band patterns or staining.
In the case of flow cytometry, a 'fluorescence minus one' (FMO) control would typically be performed, where all elements are included as usual, excluding the reagent being used to establish the threshold. Isotype controls offer a reliable alternative, provided that they exhibit the same level of non-specific binding as the antibody being utilized in your experimental design.
Inclusion of this control is particularly critical for any in vitro or in vivo blocking, neutralization, depletion, or activation assessments in which a phenotypic alteration is the intended outcome.
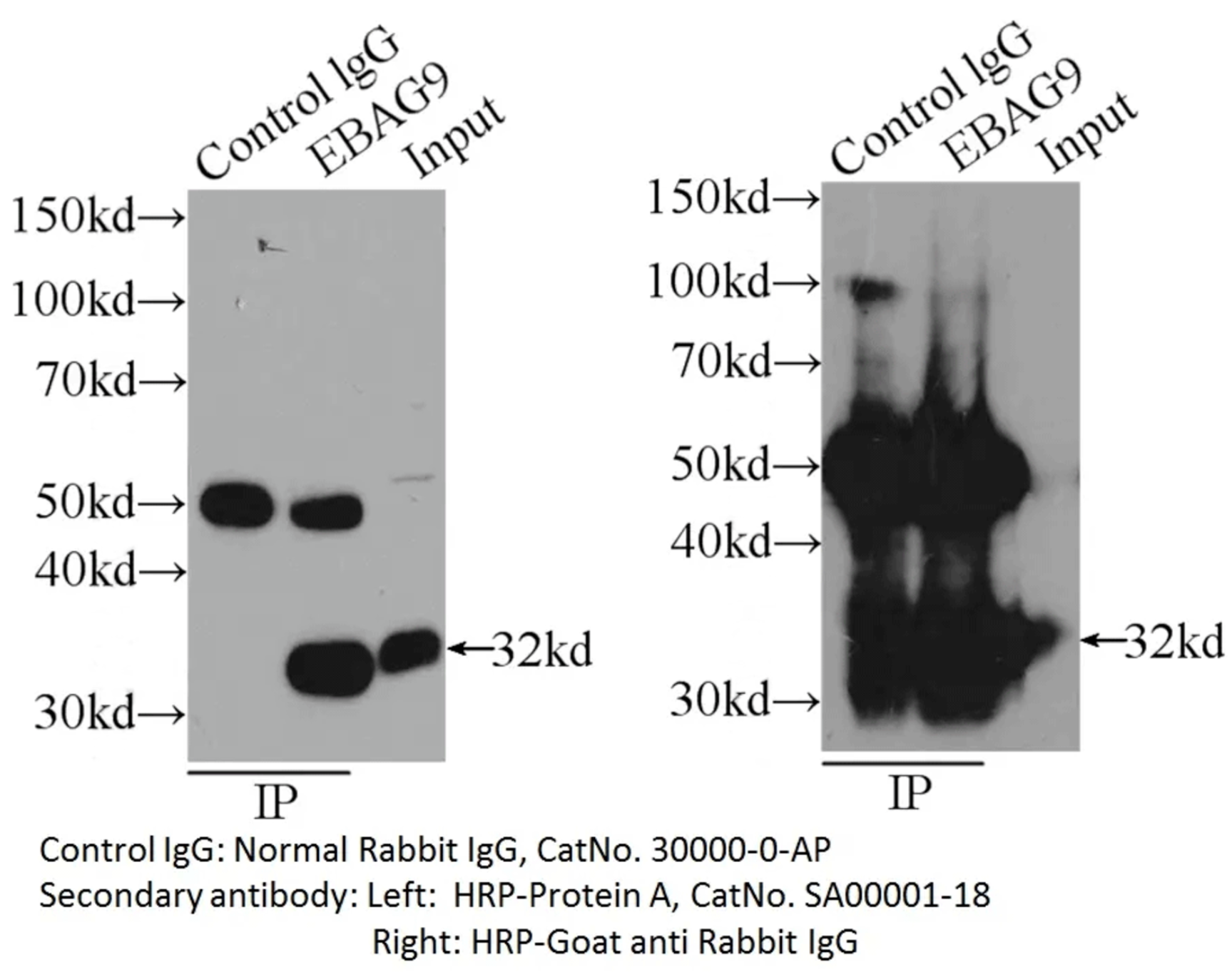
Image 3: IP sample detected with different secondary antibodies. Normal rabbit IgG (30000-0-AP) as control.
How do I choose the best Isotype Control?
To ensure effectiveness, your primary antibody and its isotype control must have several key similarities:
Same host species
Same Ig class
Same Ig subclass (for some species)
Same label as primary antibody (biotin, HRP, fluorophore, etc.)
If you utilize a mouse IgG2b antibody conjugated to FITC as a primary antibody, choosing a mouse IgG2b isotype control antibody that is also conjugated to FITC is recommended.
To determine non-specific binding of the primary antibody when employing an unconjugated primary antibody and a secondary antibody for detection, your isotype control should match your primary antibody. A control sample stained solely with secondary antibody, devoid of any primary antibody, can be incorporated to identify any secondary antibody-induced background staining.
Image 4: Standardized controls offered by Absolute Antibody
New FITC-conjugated Isotype Control
It is crucial for a control reagent to closely match with the experimental antibody used. Alomone Labs' new isotype controls were labelled using the same protocols as the regular conjugated antibodies, ensuring that the isotype control shares the same fluorophore to protein ratio (F:P ratio) as any other conjugated antibody from Alomone Labs (5-10 fluorophore molecules per antibody molecule). These isotype controls also undergo the same purification process after labelling to ensure standardisation.
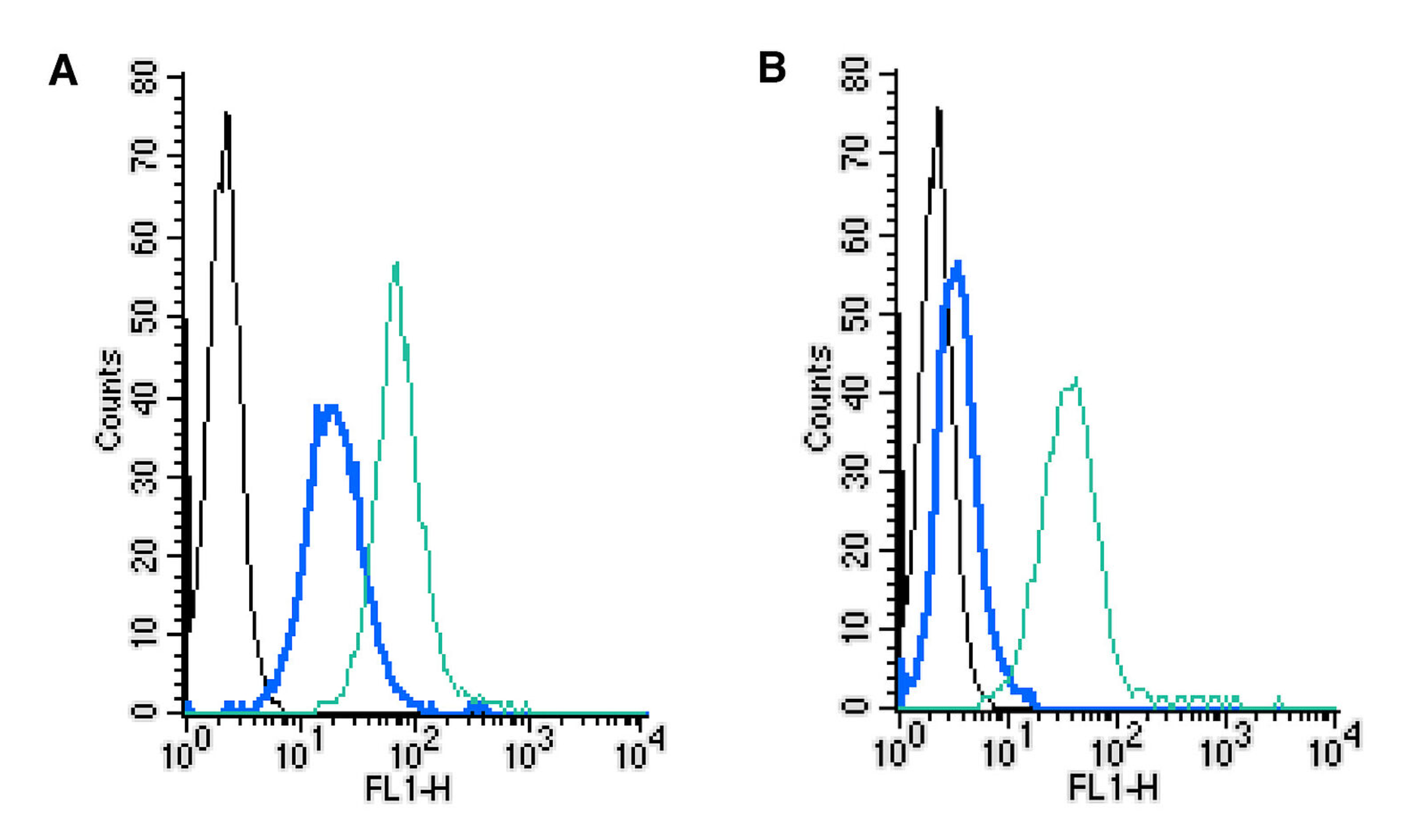
Image 5: Establishing the effectiveness of the Rabbit IgG Isotype Control-FITC (#RIC-001-F) control alongside cell surface detection of MERTK by direct flow cytometry in a live intact mouse J774 macrophage cell line. (A) The absence of an Fc block. (B) The presence of an Fc block. Black: unstained cells. Blue: cells + Rabbit IgG Isotype Control-FITC (#RIC-001-F), (2.5µg). Green: cells + Anti-MERTK (extracellular)-FITC Antibody (#ATR-033-F), (2.5µg).
References
1 Maecker, H.T., Trotter, J., 2006. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A 69, 1037–1042.
2 Perfetto, S.P., Ambrozak, D., Nguyen, R., Chattopadhyay, P., Roederer, M., 2006. Quality assurance for polychromatic flow cytometry. Nat Protoc 1, 1522–1530.
3 Cossarizza, A., Chang, H. D., Radbruch, A., Acs, A., Adam, D., Adam-Klages, S., ... & Bacher, P. (2019). Guidelines for the use of flow cytometry and cell sorting in immunological studies. European journal of immunology, 49(10), 1457-1973.
4 Roederer, M., Nozzi, J. L., & Nason, M. C. (2011). SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A, 79(2), 167-174.
Aladdin:https://www.aladdinsci.com
List of related products