Diversinates™: Any-Stage Functionalization of (Hetero)aromatic Scaffolds
Product Manager:Nick Wilde
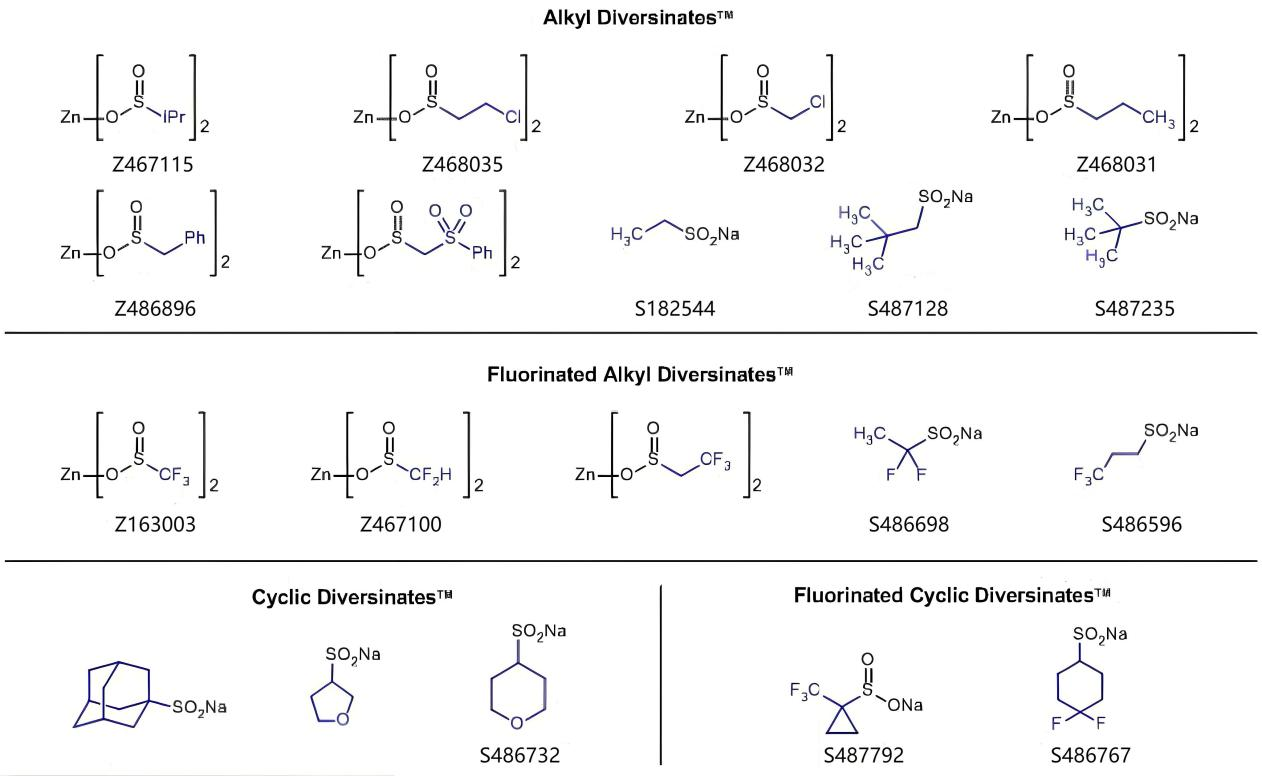
Introduction
The core of the chemical industry revolves around the synthesis of heteroaromatic and aromatic compounds. Given the escalating demand for novel chemical entities amidst dwindling resources and tight timelines imposed on research projects, there is a pressing need for a swift method to diversify (hetero)aromatic scaffolds. With the aim of simplifying operations (via reactions impervious to air and moisture), a suite of reagents has been devised, capable of efficiently appending diverse, valuable functionalities to (hetero)aromatic cores. Diversinates™, a game-changer, allow for the direct functionalization of heteroaromatic C-H bonds, bypassing the prerequisite of pre-functionalization, thereby significantly expediting the chemist's discovery process.
Advantages
Despite the robustness and indisputable usefulness of metal-catalyzed cross-coupling chemistry, it necessitates the utilization of pre-functionalized starting materials, often featuring halogenation. Conversely, metal-catalyzed C-H activation has garnered significant research attention in recent times, yet realizing (fluoro)alkylation of heteroaromatic scaffolds through this approach remains a formidable task. The innovative Baran Diversinates™ present a groundbreaking solution, capable of engaging with (hetero)arene cores devoid of obvious chemical handles, requiring solely the presence of heteroaromatic C-H bonds. These remarkable reagents harness the inherent reactivity of the substrate, facilitating reactions under practical, ambient conditions that do not necessitate the exclusion of air or moisture.
Representative Applications
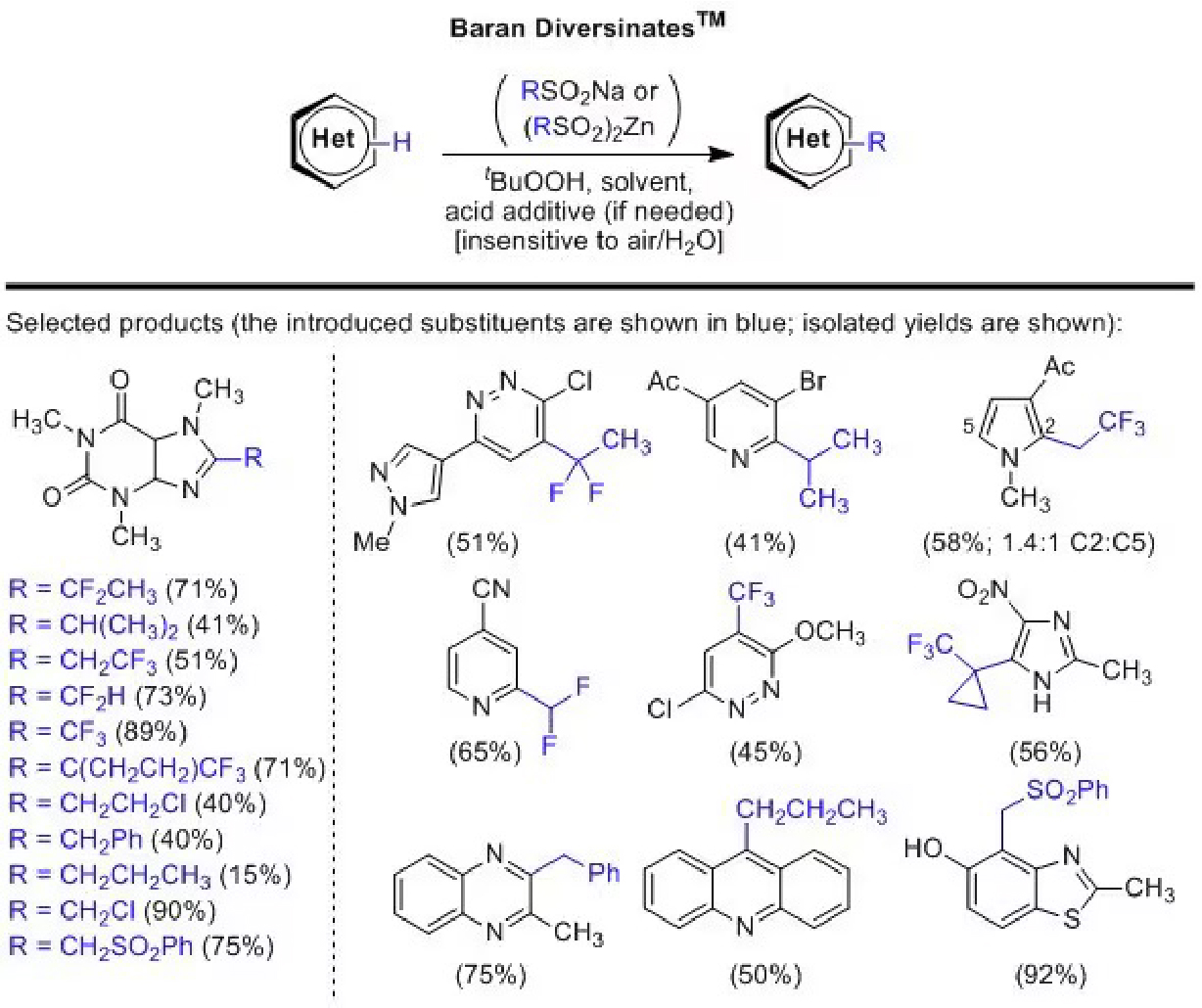
Figure 1.Representative Applications
References
1. Fujiwara Y, Dixon JA, Rodriguez RA, Baxter RD, Dixon DD, Collins MR, Blackmond DG, Baran PS. 2012. A New Reagent for Direct Difluoromethylation. J. Am. Chem. Soc.. 134(3):1494-1497. https://doi.org/10.1021/ja211422g
2. Fujiwara Y, Dixon JA, O’Hara F, Funder ED, Dixon DD, Rodriguez RA, Baxter RD, Herlé B, Sach N, Collins MR, et al. 2012. Practical and innate carbon-hydrogen functionalization of heterocycles. Nature. 492(7427):95-99. https://doi.org/10.1038/nature11680
3. Zhou Q, Ruffoni A, Gianatassio R, Fujiwara Y, Sella E, Shabat D, Baran PS. 2013. Direct Synthesis of Fluorinated Heteroarylether Bioisosteres. Angew. Chem. Int. Ed.. 52(14):3949-3952. https://doi.org/10.1002/anie.201300763
4.Gui J, Zhou Q, Pan C, Yabe Y, Burns AC, Collins MR, Ornelas MA, Ishihara Y, Baran PS. 2014. C-H Methylation of Heteroarenes Inspired by Radical SAM Methyl Transferase. J. Am. Chem. Soc.. 136(13):4853-4856. https://doi.org/10.1021/ja5007838
5.Gianatassio R, Kawamura S, Eprile CL, Foo K, Ge J, Burns AC, Collins MR, Baran PS. 2014. Simple Sulfinate Synthesis Enables C-H Trifluoromethylcyclopropanation. Angew. Chem. Int. Ed.. 53(37):9851-9855. https://doi.org/10.1002/anie.201406622
Aladdin:https://www.aladdinsci.com
