Ascorbic Acid; Vitamin C
Sandra Forbes
Product Manager

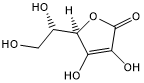
Name Reactions

Recent Literature
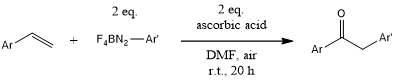
Aryl radicals generated in situ from arene diazonium fluoroborates promoted by ascorbic acid enable a convenient and general oxidative arylation of vinyl arenes in air at room temperature in the absence of any additive and visible light irradiation. Various 2-aryl acetophenones have been obtained in good yields.
B. Majhi, D. Kundu, B. C. Ranu, J. Org. Chem., 2015, 80, 7739-7745. 10.1021/acs.joc.5b00825
A cyanomethyl radical species generated from a cyanomethylphosphonium ylide by irradiation with visible light in the presence of an iridium complex, a thiol, and ascorbic acid enables an efficient 1,2-hydro(cyanomethylation) of alkenes. The cyanomethyl radical species adds across the C=C double bond to form an elongated alkyl radical species that accepts a hydrogen atom from the thiol.
T. Miura, D. Moriyama, Y. Funakoshi, M. Murakami, Synlett, 2019, 30, 511-514.
In situ nitrosation of anilines followed by reduction with ascorbic acid to form aryl radicals and thiolation with disulfides provided aryl sulfides. This mild, metal-free synthesis of aryl sulfides proceeded smoothly without heating or irradiation. This strategy can be expanded to the synthesis of aryl selenides.
M.-j. Bu, G.-p. Lu, C. Cai, Synlett, 2015, 26, 1841-1846. 10.1055/s-0034-1378738
A transition metal/ligand-free disulfuration of anilines can be performed under mild conditions and exhibits broad scope across the aniline substrate and disulfur transfer reagent classes (dithiosulfonate or tetrasulfide). This reaction can be considered as a reductive disulfuration variation of the classic Sandmeyer reaction.
S. Chen, S. Cao, C. Liu, B. Wang, X. Ren, H. Huang, Z. Peng, X. Wang, Org. Lett., 2021, 23, 7428-7433. 10.1021/acs.orglett.1c02636
An iridium photocatalyst enables the addition of fluorinated groups to nitrones using ascorbic acid as a stoichiometric reducing agent. Partially fluorinated alkyl iodides can also be effectively used. The resulting hydroxylamines can be readily converted to valuable fluorinated amines by reduction with zinc.
V. I. Supranovich, V. V. Levin, M. I. Struchkova, A. D. Dilman, Org. Lett., 2018, 20, 840-843. 10.1021/acs.orglett.7b03987
A dual cobalt- and photoredox-catalyzed HAT hydroarylation of alkenes offers high efficiency in the synthesis of a δ-lactams and various other benzo-fused hetercycles compared to established protocols. The proposed mechanism is supported by experiments and DFT calculations.
Y. Yamaguchi, Y. Seino, A. Suzuki, Y. Kamei, T. Yoshino, M. Kojima, S. Matunaga, Org. Lett., 2022, 24, 2441-2445. 10.1021/acs.orglett.2c00700
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/ascorbic-acid-vitamin-c.shtm
Aladdinsci: https://www.aladdinsci.com/





