Bioactive Compounds and Materials Based on 1,3,4-Oxadiazoles Derivatives
Introduction
It is well known that 1,3,4-Oxadiazoles are an important class of heterocyclic compounds and common heterocyclic building blocks in pharmaceutical molecules as well as in organic synthesis.[1] 1,3,4-Oxadiazole heterocycles are derivatives of furans in which two methylene groups are replaced by two nitrogen atoms. Substitution of these two methylene groups with two nitrogen atoms reduces the aromaticity of the ring and the oxadiazole ring obtained from the market exhibits conjugated diene properties. The other heteroatom forms a weak base for the oxadiazole due to induction. In nucleophilic substitution reactions, the hydrogen atom is replaced by a nucleophilic reagent.
Synthesis
The common synthetic routes for 1,3,4-oxadiazole include the reaction of acid hydrazine(or hydrazine) with chloro/carboxylic acids and the use of various dehydrating agents such as phosphorus trichloride, thionyl chloride, phosphorus pentoxide, trifluoromethic anhydride, direct cyclization of diacyl hydrazines with polyphosphoric acid, and the direct reaction of the acid with (N-isocyanamino)triphenylphosphorane.
Application
Pharmaceutical Chemistry
Many 1,3,4-oxadiazole derivatives are widely present in drug molecules due to their favorable biological and pharmacological activities [2], such as anticancer activity [3], various enzyme inhibitors [4], herbicides [5], and antifungal activity [6]. The already marketed HIV antiviral drug, Raltegravir (Raltegravir) 1 , exhibits potent inhibition of the strand transfer process catalyzed by HIV integrase to maximize their inhibitory effects as HIV integrase inhibitors against wild-type viruses and some mutants, and to optimize their selectivity, pharmacokinetic and metabolic profiles in preclinical species. [7] Zibotentan 2 is a specific ETA receptor antagonist currently in clinical development for the treatment of hormone-resistant prostate cancer (HRPC). [8] As well as the marketed herbicide Methoxydiazone 3 (Fig. 1) has the structural unit of 1,3,4-oxadiazole in its chemical structure, which is a good indication of the potential of the 1,3,4-oxadiazole heterocycle for drug discovery and agrochemistry applications.
Materials Chemistry
In addition to this, 1,3,4-oxadiazoles have been widely used for the fabrication of novel materials. For example, heat-resistant polymers based on 1,3,4-oxadiazole structures [9], alternating copolymers of 9,9-dioctylfluorene and oxadiazole prepared using the tetrazole route or the Suzuki coupling reaction, which can be used as electron-transporting and blue-light-emitting polymers (e.g., polymer 4 in Fig. 1) [10], dendritic polymers consisting of 1,3,4-oxadiazole repeating units [11], efficient laser dyes [12] and metal liquid crystals [13].
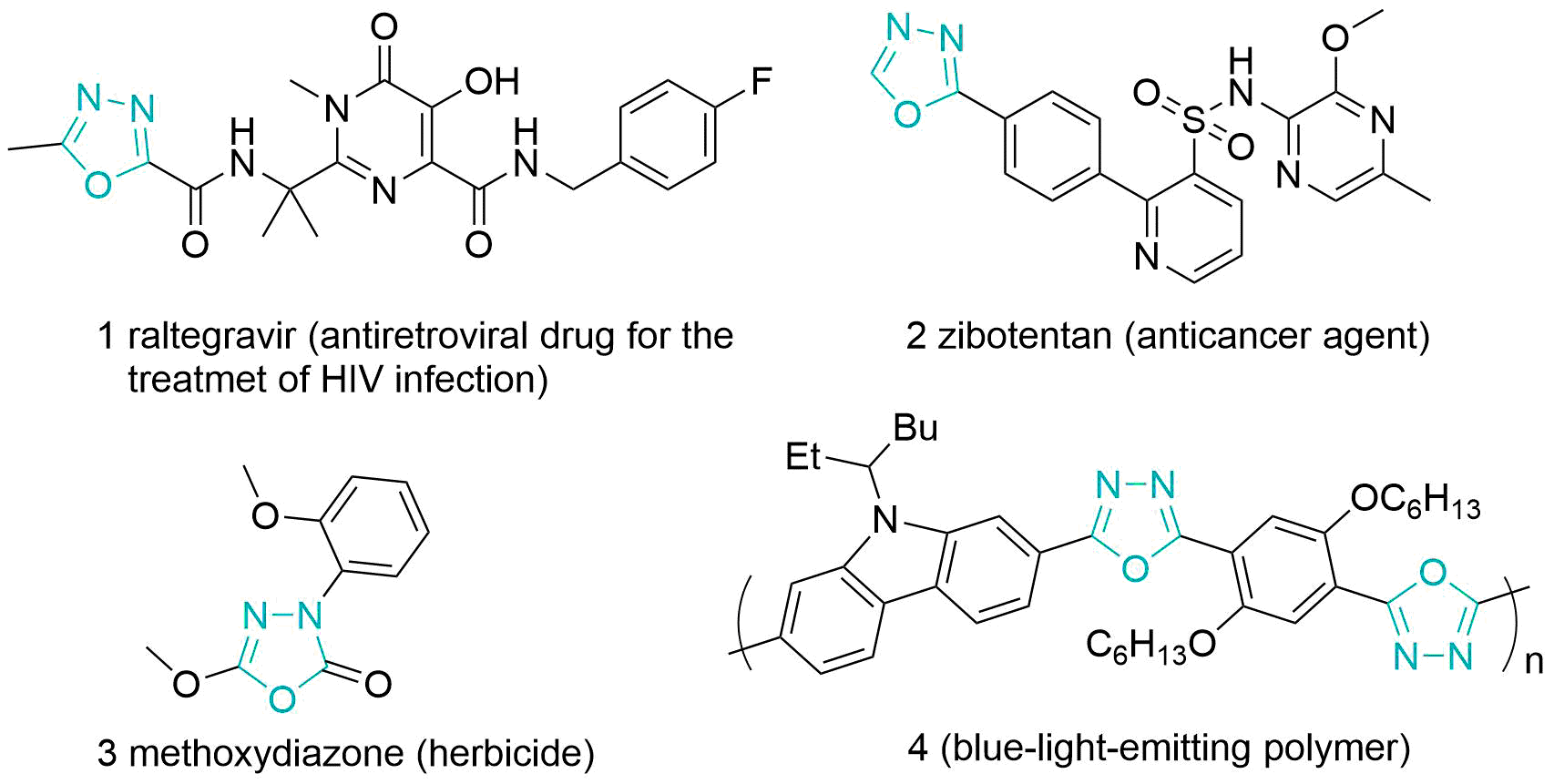
Fig 1. Examples of 1,3,4-oxadiazole-based bioactive compounds and materials
Organic Chemistry
1,3,4-Oxadiazoles have long been widely used in organic chemical synthesis for the generation of highly reactive carbene intermediates reacting with electrophilic functional groups [14].2,5-Dihydro-1,3,4-oxadiazoles play an important role in organic synthesis due to their ease of preparation and considerable stability, and are also a source of pyrogens for bis(heteroatomic) alkene carbenes. Such allen-carbons react with a variety of electrophilic functional groups, usually leading to rearrangements of the initial products. These products can be used as starting materials for other synthetic targets, while the by-products of their thermal decomposition are mainly N2 and ketones, making the isolation of the products from the reaction relatively straightforward.Another promising synthetic application of 1,3,4-oxadiazoles is their intramolecular [4+2]/[3+2] cross-cycloaddition reactions [15].
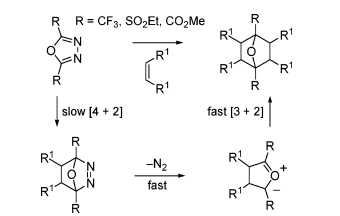
Fig 2. Examples of 1,3,4-oxadiazoles involved in intermolecular reaction cascades
Reference
1. J. Suwiński, J.; Szczepankiewicz, W. in Comprehensive Heterocyclic Chemistry, 3rd edition, eds. A.R. Katritzky, C.A. Ramsden, E.F.V. Scriven, and R. J.K. Taylor, Pergamon, Oxford, 2008. Vol. 5, p. 398.
2. Yogesh, M.; Senthilkumar, G. P. World J. Pharmaceut. Res. 2019, 8, 1406-1428.
3. Glomb, T.; Szymankiewicz, K.; Swiatek, P. Molecules 2018, 23, 3361/1-3361/16. https://doi.org/10.1021/acsomega.2c01586
4. Boström, J.; Hogner, A.; Llinàs, Wellner, E.; Plowright, A.T. J. Med. Chem. 2012, 55, 1817. https://doi.org/10.1016/j.bmcl.2015.11.046
5. Das, A.C.; Debnath, A.; Mukherjee, D. Chemosphere 2003, 53, 217. https://doi.org/10.1016/s0045-6535(03)00440-5
6. Zou, X.-J.; Lai, L.-H.; Jin, G.-Y.; Zhang, Z.-X. J. Agric. Food Chem. 2002, 50, 3757. https://doi.org/10.1107/S241431462200342X
7. Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M. et al. J. Med. Chem. 2008, 51, 5843. https://doi.org/10.1021/jm800245z
8. James, N. D.; Growcott, J. W. Drugs Future 2009, 34, 624.
9. Hill, J. in Comprehensive Heterocyclic Chemistry, 2nd edition, eds. A. R. Katritzky, C. W. Rees, and E. F. V. Scriven, Pergamon, Oxford, 1996, vol. 4, p. 268.
10. Ding, J.; Day, M.; Robertson, G.; Roovers, J. Macromolecules 2002, 35, 3474. https://doi.org/10.1021/ma011093n
11. Verheyde, B.; Dehaen, W. J. Org. Chem. 2001, 66, 4062. https://doi.org/10.1002/pola.21333
12. Nijegorodov, N.; Mabbs, R. Spectrochim. Acta, Part A, 2002, 58, 349. https://doi.org/10.1016/s1386-1425(01)00544-3
13.Wen, C.-R.; Wang, Y.-J.; Wang, H.-C.; Sheu, H.-S.; Lee, G.-H.; Lai, C.K. Chem. Mater. 2005, 17, 1646.
14. Warkentin, J.; Acc. Chem. Res. 2009, 42, 205. https://doi.org/10.1021/ar800072h
15. Elliott, G.I.; Fuchs, J.R.; Blagg, B.S.J.; Ishikawa, H.; Tao, H.; Yuan, Z.-Q.; Boger, D.L. J. Am. Chem. Soc. 2006, 128, 10589. https://doi.org/10.1021/ja0612549
