Bis(pinacolato)diboron, B2pin2
Sandra Forbes
Product Manager
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula [(CH3)4C2O2B]2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making pinacol boronic esters for organic synthesis. Unlike some other diboron compounds, B2pin2 is not moisture-sensitive and can be handled in air.

Recent Literature

CrCl2 mediates a nucleophilic addition of a primary amine derivative to an aldehyde to provide an alcohol. This radical-polar crossover process is selective for aldehydes and compatible with numerous functional groups, which are not tolerated under classical Grignard-type conditions.
Y. Huang, Z. Liu, W. H. Liu, Org. Lett., 2023, 25, 4934-4939.
DOI: 10.1021/acs.orglett.3c01724

A Pd-catalyzed reaction of water with a diboron compound as the reductant produces clean hydrogen gas under ambient reaction conditions. The B2Pin2-H2O system enables a selective hydrogenation of olefins in the presence of a palladium catalyst.
D. P. Ojha, K. Gadde, K. R. Prabhu, Org. Lett., 2016, 18, 5062-5065.
DOI: 10.1021/acs.orglett.6b02508

A B2Pin2-assisted copper-catalyzed semihydrogenation of alkynes provides various alkenes in good to excellent yields with Z-selectivity under mild reaction conditions. The present protocol enabled convenient synthesis of deuterium-substituted Z-alkenes using readily available ethanol-d1 as the deuterium source.
H. Bao, B. Zhou, H. Jin, Y. Liu, J. Org. Chem., 2019, 84, 3579-3589.
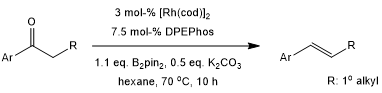
Rhodium-catalyzed deoxygenations and borylations of ketones with B2pin2 provide alkenes, vinylboronates, and vinyldiboronates. These reactions offer mild reaction conditions, a broad substrate scope, and excellent functional-group compatibility. Mechanistic studies support that the ketones initially undergo a Rh-catalyzed deoxygenation to give alkenes via boron enolate intermediates.
L. Tao, X. Guo, J. Li, R. Li, Z. Lin, W. Zhao, J. Am. Chem. Soc., 2020, 142, 18118-18127.
DOI: 10.1021/jacs.0c07854
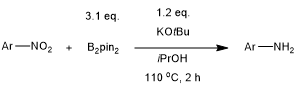
The combination of B2pin2 and KOtBu enables a chemoselective, metal-free reduction of aromatic nitro compounds to the corresponding amines in very good yields in isopropanol. The reaction tolerates various reducible functional groups.
H. Lu, Z. Geng, J. Li, D. Zou, Y. Wu, Y. Wu, Org. Lett., 2016, 18, 2774-2776.
DOI: 10.1021/acs.orglett.6b01274
A visible-light-promoted transfer hydrogenation of azobenzenes proceeds smoothly in methanol at ambient temperature in the presence of B2pin2 through a radical pathway. The reaction reduces a broad range of azobenzenes to the corresponding hydrazobenzenes in very good yields.
M. Song, H. Zhou, G. Wang, B. Ma, Y. Jiang, J. Yang, C. Huo, X.-C. Wang, J. Org. Chem., 2021, 86, 4804-4811.
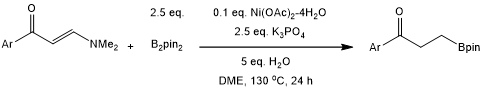
An unprecedented nickel-catalyzed reductive borylation of enaminones provides β-ketone boronic esters via a challenging transformation of an alkenyl C(sp2)-N bond. While B2pin2 plays a dual role in this process, water serves as the hydrogen source.
X. Li, Z. Chen, Y. Liu, N. Luo, W. Chen, C. Liu, F. Yu, J. Huang, J. Org. Chem., 2022, 87, 10349-10358.
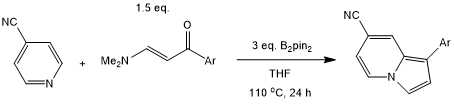
B2pin2 mediates a radical cascade cyclization/aromatization reaction of enaminone with pyridine to provide valuable functionalized indolizines under metal-, external oxidant-, and base-free conditions. The reaction is compatible with a broad range of functional groups, such as halogens, π-systems, and heterocycles.
W.-W. Ding, Y. Zhou, S. Song, Z.-Y. Han, Org. Lett., 2022, 24, 7350-7354.
DOI: 10.1021/acs.orglett.2c02905
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/bis(pinacolato)diboron-B2pin2.shtm
Aladdinsci: https://www.aladdinsci.com/