Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
New Synthesis Method of New Nickel Reagent and Boric Acid (Ester)

Boric acid (ester) is an important molecular synthesis block, especially special boric acid (ester) such as heterocyclic ring, allyl, saturated hydrocarbon and substrate containing two functional groups, are popular blocks in synthetic chemistry.
In 2019, JACS published an article by Professor Melanie S. Sanford describing the method of synthesizing boric acid (ester) directly from acid, which is illustrated as follows:
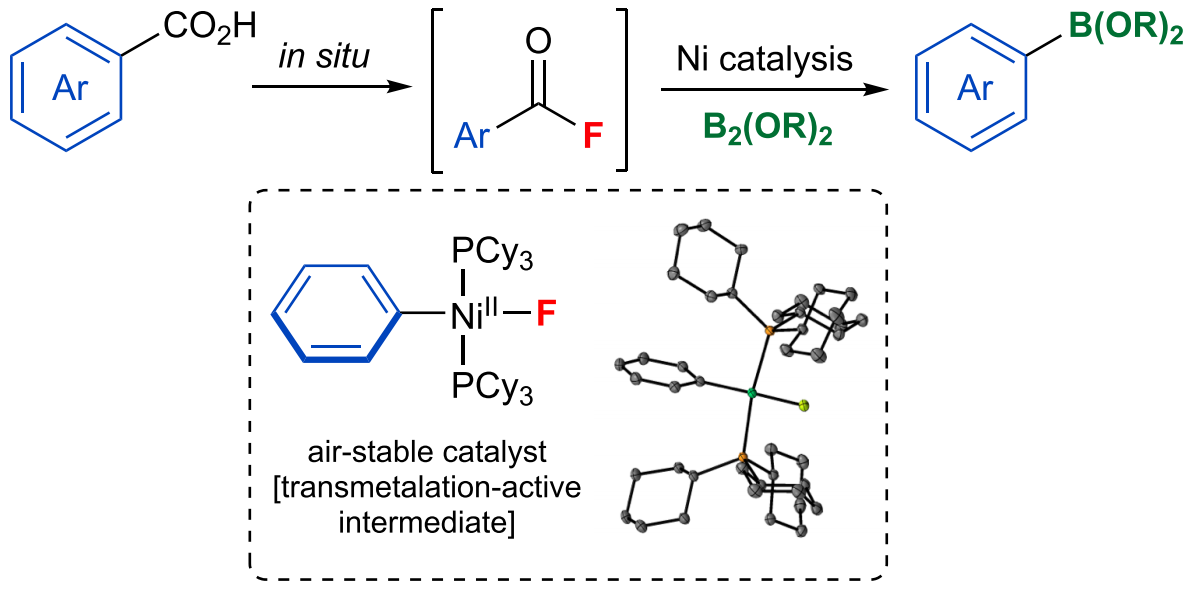
JACS 2019 Oct 16. doi: 10.1021/jacs.9b08961
Advantages of this method
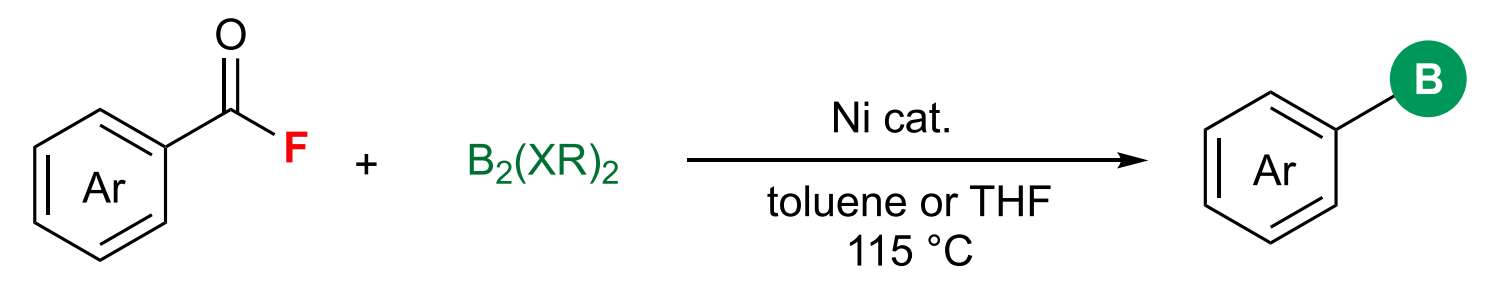
Ni-catalyzed decarbonylative borylation of carboxylic acid fluorides
• base-free conditions, highly selective towards borylation
• general, compatible with various boronic esters and boronamides
• abundant carboxylic acids as substrates
• trans-metalation-active intermediate as an air-stable catalyst
• mechanistic studies present trends in trans-metalation reactivities of Ar-Ni-F complexes with organoboron and diboron reagents
Experimental method
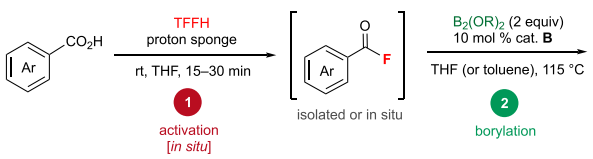
General procedure for decarbonylative borylation reaction (in situ, from carboxylic acid): In a nitrogen-filled glovebox, carboxylic acid (0.3 mmol, 1 equiv), TFFH (0.3 mmol, 1 equiv), and proton sponge (0.3 mmol, 1 equiv) were weighed into a 10 mL tall vial equipped with a 10 μm magnetic stir bar. THF (0.5 mL) was added, and the reaction mixture was stirred at room temperature for 15 to 30 min. Catalyst B (0.03 mmol, 0.1 equiv) and diboron reagent (0.6 mmol, 2 equiv) were added. The vial was capped and removed from the glovebox. The reaction mixture was stirred at 115 oC for 16 h. The reaction was then cooled to room temperature, and Et2O (10 mL) and saturated NaHCO3 (10 mL) were added. The organic layer was collected, and the aqueous solution was further extracted with Et2O (2×10 mL). The combined organic extracts were dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel using EtOAc in hexanes.
Examples of reactions
Building blocks may be applicable for the method
References
1. Christian A. Malapit, James R. Bour, Simon R. Laursen, and Melanie S. Sanford, J. Am. Chem. Soc. 2019, 141, 43, 17322–17330. https://doi.org/10.1021/jacs.9b08961
Aladdin:https://www.aladdinsci.com |
- Help & Customer Service
- FAQs
- What do the terms MSDS, SDS, COA, and TDS in the chemical industry mean? How do they differ?
- Why are our chemicals so much less expensive than the same chemicals sold by your competitors?
- What are MSDS and SDS?
- What are the requirements to become an Aladdin distributor?
- Are there any discounts or sales rebates for distributors?
- What is the duration of the distributor agreement?
- Will I receive training and support as a distributor?
- Under which law is the distributor agreement governed?
- The differences between registered users(with a profile) and non registered users
- Delivery and receipt specification
- What is a third -party express or logistics company delivery?
- My order was charged the freight. If the return in the later period, will the previous freight be refunded to me?
- What should I do if the goods are less/the product is wrong?
- What should these products be used for and who should use them?
- Why are chlorophosphonate liposomes useful for studying macrophage function?
- What is the CAS number?
- What dose of antagonist, agonist, or signaling tool should be used in vivo?
- Aladdin product purity and quality: what is it and how is it determined?
- What dose of an antagonist, agonist, or signaling tool should be used in vitro?
- How do I determine if a compound is cell-permeable?
- What should I do if I can't see any product in the vial?
- Should different batches of a product look the same?
- What is the molecular weight and molecular formula?
- How do I dissolve my product?
- How should I store my product?
- Effects of storage on solubility
- Why does Aladdin‘s Biochemicals sometimes ship chemicals at room temperature when the vial is labelled 'store at +4°C or -20°C'?
- What is the half-life of clodronate (liposomes)?
- Pack sizes and weighing accuracy: do I need to reweigh my product?
- I did not observe macrophage depletion, what can be the reason?
- My animal has died immediately after injection of (clodronate) liposomes。
- How to know how much peptide is in my vial?
- My animal has died several hours or days after injection of (clodronate) liposomes.
- How should I store and use lyophilized peptides?
- How should l store the liposomal suspension?
- What is the concentration of the clodronate liposome suspension?
- How should I dissolve and store peptide solution?
- What is the size and charge of the liposomes?
- ln which medium are the liposomes suspended?
- Dissolved amino acids: how to prepare a solution with 1 equivalent of NaOH?
- How can l detect the Dil-labeled liposomes?
- What types of experiments are our products eligible for?
- I need the SDS and Certificate of Analysis for the product that I purchased.
- Where can I get protocols for using your products?
- GHS Classification
- How to obtain a quality inspection certificate (COA)?
- Does aladdin test for endotoxin in their antibodies?
- What should I know about the stability of your protein products?
- Which isotype are Aladdin’s polyclonal antibodies?
- What endotoxin level should be expected when purchasing Aladdin proteins?
- How are your antibodies purified?
- Why can’t I see the protein pellet in the vial?
- Can you tell me what epitope your antibody binds to?
- Which cytokines show cross-species activity?
- Have Aladdin’s antibodies been tested in neutralization assays?
- What is the relationship between the specific activity expressed as an ED50 and as units/mg?
- Are Aladdin’s antibodies suitable for use in ELISA and Western Blot applications?
- What information should be known about the stability of your antibody products?
- Do Aladdin’s antibody products contain any carrier proteins or other additives?
- What is the relationship between specific activity units and International Units of activity?
- How does Aladdin obtain International Units of activity?
- Will Aladdin antibodies work in immunohistochemistry and immunocytochemistry applications?
- Will Aladdin antibodies recognize target proteins sold by other vendors?
- Will Aladdin antibodies recognize target protein in complex biological fluids such as blood or serum?
- What are the differences between encapsulation efficiency, loading capacity, and yield?
- How can micelle stability be improved?
- Can nanoparticles be used for oral drug delivery?
- What is the ideal size for a nanocarrier?
- What strategies can be used for the extended release of peptides for one month or more?
- When considering PEGylation for a protein drug, how do you decide what PEGylation chemistry to use?
- What are Building Blocks?
- What are the applications of Building Blocks in drug development?
- What are the common types of drug Building Blocks?
- What are the substituted functional groups of the Building Blocks?
- What role do Building Blocks play in new drug development?
- Which Building Blocks are more popular in medicinal chemistry?
- What are the main competition barriers of molecular Building Blocks industry?
- What is the application scale of molecular Building Blocks in drug development?
- What are the targets of molecular Building Blocks?
- What are the synthetic techniques of molecular Building Blocks?
- My DMSO-d6 appears to be a solid. What can I do?
- How can I measure acidity levels in D2O solutions?
- Should my NMR solvents be handled in a special way?
- Does my isotope solvent require special storage?
- What are the 3 most commonly purchased isotopes in trace element analysis and their quantity of supply?
- What type of mineral oil is contained in the 6Li metal, and how would this be removed?
- What are the key criteria for selecting stable isotope-labeled standards for clinical measurements?
- What is meant by descriptions such as TC treated/no TC treated that appear in product descriptions such as cell culture dishes?
- Which surface should I use to grow suspension cells?
- How should closed-cap cell culture flasks be used? What is the difference between the use of the lid with the filter membrane?
- What do we often hear about T25 or T175?
- I used i-Quip® surface treated culture bottle/dish/plate, the cells do not adhere to the surface, is it a product quality problem?
- After removal from the liquid nitrogen tank, the cell cryogenic vials will occasionally burst. How to avoid it?
- What precautions should be taken when using cell cryogenic vials?
- What are the possible reasons for the cracking phenomenon of the centrifuge tube during centrifugation?
- Why is it necessary to add carrier protein to some recombinant protein solutions?
- Will glycosylation affect the biological activity of recombinant protein?
- How to operate the reconstitution and preservation of recombinant protein?
- How can I save the recombinant protein after re-dissolution?
- What are the main differences between the recombinant proteins produced by different expression systems?
- Why didn't I detect the activity of the recombinant protein in the experiment?
- Can recombinant proteins of different species be cross-used?
- What is the role of the protein tag? Does the fusion tag need to be removed during the experiment?
- Is it possible to use the vortex shaker to help the lyophilized powder fully dissolve?
- What is Friedel Craft reaction with example?
- What are the advantages of Friedel Crafts acylation?
- Is Friedel-Crafts alkylation reversible?
- How is a Lewis acid used in Friedel Crafts acylation?
- What is alkylation of benzene?
- Why does nitrobenzene not undergo the Friedel-Crafts acylation reaction?
- What are the limitations of Foucault alkylation reaction?
- What are the limitations of the Foucault acylation reaction?
- What is COA?
- Distinguishing between MSDS, COA, and TDS?
- How can I get a trial size product for free?
- Is there a limit to how many free trial size products I can receive?
- Will I have to pay anything for the trial size product?
- How will I receive the discount coupon?
- Can the discount coupon be used for any product?
- How do I apply to become an Aladdin distributor?
- What is the process after submitting the distributor application?
- Can I return products? What is the return policy?
- What happens if either party wants to terminate the agreement?
- Is there a confidentiality clause in the distributor agreement?
- What if I encounter issues using my discount coupon?
- What types of targets are there?
- read more
- Technical articles
- Strain-promoted alkyne-nitrone cycloaddition (SPANC)
- DEAE-Dextran
- IgG
- Dextran Chemistry
- liposomes
- Hyaluronan
- Macrophage Stimulating Protein (MSP)
- Click Chemistry
- BINOL and Derivatives
- Linkers - A Crucial Factor in Antibody–Drug Conjugates
- Karl Fischer Titration to Measure the Water Content of Samples that Do not Readily Release Water
- Application of Gold Catalysts in Industrial Hydrogenation Process
- N-Heterocyclic Carbene (NHC) Ligands
- Applications of Nanoparticles in Pathogen Detection and Identification
- Cannizzaro Reaction
- Clemmensen Reduction
- Guide to Adherent Cell Culture Basics: Seeding, Expanding, and Harvesting
- Application of Granular Materials in Immunoassay
- Properties and Applications of Magnetic Nanoparticle
- Wolff-Kishner Reduction
- Fries rearrangement
- Sulfonyl Chlorides and Sulfonamides
- Alzheimer’s Disease Signaling
- DNA Damage and Repair
- How To Improve the Safety of Electrolyte in Lithium-Ion Batteries?
- Research Progress of High-Voltage Lithium-Ion Battery Materials
- Application of Magnetic Nanoparticles in Protein Expression
- Silica-Coated Gold Nanoparticles: Surface Chemistry, Properties, Benefits, and Applications
- Application of albumin
- Friedel–Crafts Acylation
- Immunoprecipitation technology
- Aldol Condensation Reaction
- Basic Concepts about Catalysts
- Baeyer-Villiger Oxidation
- Cinchona Alkaloids
- Application of Magnetic Particles in Organelle Separation
- Nicewicz Photoredox Catalysts for Anti-Markovnikov Alkene Hydrofunctionalization
- Application of Magnetic Nanoparticles in RNA and DNA Separation
- Homobenzotetramisole (HBTM): A General Organocatalyst for Asymmetric Acylations
- Nanoparticle-Based Small Molecule Drug Delivery
- Visible Light Photoredox Catalysts
- Applications of Nanoparticles in Vaccine Delivery
- Markovnikov’s Rule
- Grignard Reagent
- Neurotransmitters, Receptors, and Transporters
- Hydrogenation Catalysts
- Organic Photoredox Catalysts for Visible Light-Driven Polymer and Small Molecule Synthesis
- Knoevenagel Condensation Reaction
- Grignard reaction
- Nanoparticle-Based Gene Delivery
- Regulation of TGF-beta activity by BMP-1
- Retinoic Acid and Gene Expression
- Guide to Sialylation: I Neu5Ac and Neu5Gc Quantitation
- Guide to Sialylation: II Highly Sialylated Glycoproteins
- Quantitative Sialic Acid Analysis
- SuFEx: Sulfonyl Fluorides that Participate in the Next Click Reaction
- Reductive Amination with 2-picoline-borane Complex
- Copper-Free Click Chemistry
- Mesoporous Materials: Properties and Applications
- Media and Supplements in Cell Culture
- Quantum dots
- Materials for Advanced Thermoelectrics
- Application of Graphene in Photocatalysis
- Silver Nanomaterials for Biological Applications
- Stripping and Reprobing Western Blotting Membranes
- Graphene Inks for Printed Electronics
- Procainamide Labeling Kit-2-Picoline Borane-24T
- Procainamide Labeling Kit-Sodium Cyanoborohydride-24T
- Procainamide Labeling Kit-Sodium cyanoborohydride-96T
- Permethylation Kit
- 2-AB Labeling Kits-Sodium Cyanoborohydride
- 2-AB Labeling Kits-2-Picoline Borane
- Buffer Reference Center
- Poly(N-isopropylacrylamide)-based Smart Surfaces for Cell Sheet Tissue Engineering
- Polymer-Clay Nanocomposites: Design and Application of Multi-Functional Materials
- Light-emitting Polymers
- Small RNA modification: important functions and related diseases
- Conductive Polymers for Advanced Micro- and Nano-fabrication Processes
- Nucleic Acid Gel Electrophoresis—A Brief Overview and History
- Continuous-wave InAs/GaAs quantum-dot laser diodes monolithically grown on Si substrate with low threshold current densities
- Application of nucleoside pharmaceutical intermediates
- Nucleic Acid Electrophoresis Workflow—5 Main Steps
- Polyethylene Glycol (PEG) Selection Guide
- Advanced Inorganic Materials for Solid State Lighting
- Biological buffer: the unusual amongst the usual
- Applications of Fullerenes in Bioscience and Optoelectronics
- General Conjugation Protocols of PEG linkers——PEG Amine
- General Conjugation Protocols of PEG linkers——PEG Acid
- General Conjugation Protocols of PEG linkers——PEG-NHS Ester
- General Conjugation Protocols of PEG linkers——PEG Maleimide
- General Conjugation Protocols of PEG linkers——PEG Thiol
- General Conjugation Protocols of PEG linkers——PEG PFP Ester
- General Conjugation Protocols of PEG linkers——PEG SPDP
- General Conjugation Protocols of PEG linkers——PEG Aldehyde
- General Conjugation Protocols of PEG linkers——PEG ONH2
- What are the advantages of glass chromatographic column?
- Handling Method for Possible Abnormalities of D101 Macroporous Resin in Use
- Use process of combined carbonization dialysis bag
- Filling method and precautions of chromatographic column
- What are the material, dialysis power and speed of viskase dialysis bag?
- Can the ready to use dialysis bag be reused? Yes, but not recommended
- Differences between Ni IDA and Ni NTA agarose gels
- Instructions for ready to use CE membrane dialysis bag
- User Manual of Ready to use RC Membrane Dialysis Bag
- Selection of molecular weight (MWCO) and width of dialysis bag
- Construction and application of single-walled carbon nanotube networks
- Nucleotide Synthesis in Cancer Cells
- EGF Signaling: Tracking the path of Cancer
- PEG-Azide-Alkyne—Bioorthogonal and Click Chemistry
- Enzymes and dietary antioxidants
- Functional group protection and deprotection in oligonucleotide synthesis
- Nucleic Acid Electrophoresis Additional Considerations–7 Aspects
- Preparation and functionalized design of novel graphene-based nanostructures
- Calcium indicators and ionophores
- Oligonucleotide synthesis
- Nucleic acid electrophoresis applications - preparative and analytical electrophoresis
- Editing sugar chains on therapeutic proteins with glycosidase
- Popular Semiconductor Materials: Application Introduction of Gallium Arsenide
- Monosaccharide Release and Labeling Kit-96T
- Study on Alkenyl Fluorinated Building Blocks
- Silicon carbide provides technical solutions for the photovoltaic field
- Papain and its application
- Guidelines for troubleshooting nucleic acid electrophoresis
- Semiconductor material band gap know how much?
- Degradable Poly(ethylene glycol) Hydrogels for 2D and 3D Cell Culture
- 2-AA Labeling Kit-Sodium Cyanoborohydride
- Trifluoromethyl in organic synthesis
- 2-AA Labeling Kit-2-Picoline Borane
- V-Tag Glycopeptide Labeling Kit
- Exoglycosidase Clean-up Plate
- JAK-STAT Cell Signaling Pathway
- Versatile Cell Culture Scaffolds via Bio-orthogonal Click Reactions
- CRISPR/Cas9 and targeted genome editing
- BioQuant Monosaccharide Standard
- Antibody-drug Conjugates: A Comprehensive Guide
- Negative Photoresist Lithography Process
- Advanced enzyme analysis technology
- N2 Column
- Enzymatic determination of pepsin
- N1 Column
- C3 Anion Exchange Column
- C2 Strong Anion Exchange Columns
- CEX Cartridges for O-glycans
- S Cartridges
- T1 Cartridges
- Procainamide Cleanup Plate
- Biomolecular NMR: Isotope Labeling Methods for Protein Dynamics Studies
- Culture scheme of neural stem cells
- Dissociation of cells with trypsin
- Semiconductor Materials: A Summary of Questions
- PD-1/PD-L1 Signaling Pathway
- Research progress of introducing difluoromethyl
- Pre-Permethylation Clean-up Plate
- EB10 Cartridges
- EC50 Cartridges
- EC50 plate
- New Uses for Organoids
- Storage and Use Information Guide of NMR Solvents
- Research Progress of Silicon Anode Materials for High Performance Lithium-ion Batteries
- Aryl fluorination
- Research Progress of Cathode Materials for Lithium-Ion Batteries
- PANoptosis: An inflammatory programmed cell death pathway
- Efficient synthesis of Fluorinated Azaindoles
- Stable Isotope Applications
- Aladdin New Product Label——Your Reagent Usage Guide
- Small Molecule Inhibitors Selection Guide
- Fluoroalkylation: Expansion of Togni Reagents
- Common Modification Strategies for Lithium Battery Separators
- Vitamin D and the prevention of human disease
- Biological enzyme catalysis technology and application
- Hydrosilylation and Hydrosilylation Catalyst
- Stable Isotopes in Drug Development and Personalized Medicine: Biomarkers that Reveal Causal Pathway Fluxes and the Dynamics of Biochemical Networks
- Guide for the Selection of Lithium Salts in the Electrolyte of Lithium-Ion Batteries
- Metabolic signaling pathway
- Protein sample preparation process
- FAQ: Karl Fischer Reagent
- Enzyme Probes
- Conversion Lithium Metal Fluoride Batteries
- Ionic Liquid Electrolytes for Li-ion Batteries
- Allenes Building Blocks with ability to participate in multiple reactions
- Solid State Rechargeable Battery
- The Scope of Application of the Karl Fischer Method
- RNA Viruses Triggered Signal Pathway
- Heck Reaction
- Sample Preparation Method of Karl Fischer Reagent in the Pharmaceutical Industry
- Difference Between Karl Fischer Coulometry and Volumetric Method Compare the Difference Between Similar Terms
- Frequently asked Questions about inhibitors and antagonists related products(FAQ)
- Diels–Alder Reaction
- Cytokines and Inflammation
- Macrophage Activation
- Biginelli Reaction
- Macrophage Stimulating Protein (MSP)
- Nanoparticle-Based Nucleic Acid Delivery
- CXCR4: Receptor for extracellular ubiquitin
- TEMPO Catalyzed Oxidations
- Application of Colloidal Gold in Electron Microscope
- Photoredox Iridium Catalyst for Single Electron Transfer (SET) Cross-Coupling
- Application of Nanomaterials in Photoacoustic Imaging
- Applications of imidazole and its derivatives
- Asymmetric Organocatalysts
- MacMillan Imidazolidinone Organocatalysts
- Application of Nanomaterials in Wastewater Treatment
- Proline-type Organocatalysts
- 1,2,4-Triazole Derivatives for Synthesis of Biologically Active Compounds
- Chemical Synthesis Methods of Nanomaterials
- Substituted Azetidines in pharmaceutical chemistry, organic synthesis, and biochemistry
- Bioactive Compounds and Materials Based on 1,3,4-Oxadiazoles Derivatives
- Alcohol Oxidation Catalysts with Higher Activity than TEMPO——AZADOL®
- Chiral Phosphoric Acids——Versatile Organocatalysts with Expanding Applications
- Click Chemistry in Drug Discovery
- How to Prepare Graphene Quantum Dots?
- Soluble Pentacene Precursors
- A New Role for Angiotensin II in Aging
- What is TDS?
- Optimizing Quantum Dots to Maximize Solar Panel Efficiency
- Quantum Dots for Electronics and Energy Applications
- Fluorescent Probes - Absorption and Fluorescence
- Applications of Quantum Dot Technologies in Cancer Detection and Treatment
- Fluorescence Quenching
- Perovskite Quantum Dots (PQDs)
- 1,3-Thiazole building blocks in natural products and synthetic materials
- Dye-Aggregation
- Antibody structure and isotypes
- Realizing High Efficiency in Organic Light Emitting Devices
- Quantum Dots——A Definition, How They Work, Manufacturing, Applications and Their Use In Fighting Cancer
- Loading Controls for Western Blotting
- Flow Cytometry
- Derivatives of 1,3,4-thiadiazoles for Various Applications in Drug Discovery, Agrochemistry, and Materials Technology
- Flow cytometry analysis
- How to choose and use antibodies-primary antibody?
- Quantum Computing - Is it the Future?
- Inorganic Interface Layer Inks for Organic Electronic Applications
- How to find the right secondary antibody?
- Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)
- How to Choose the Correct Reference Material Quality Grade?
- Antibody applications and techniques
- Reactions of strained alkenes in click chemistry
- Strain-promoted azide-alkyne cycloaddition (SPAAC)
- Determination of Additives in Beverages
- Potential Applications in Click Chemistry
- Click Chemistry
- Fluid chemistry: Lighting the fire of hope for the large-scale production of anticancer drugs
- Magnetic Resonance Imaging
- Synthesis of molecular block “two-sided” indole
- New Synthesis Method of New Nickel Reagent and Boric Acid (Ester)
- New Breakthrough in the "Transformation" Technology of Pentane Skeleton Drugs: One-Pot Synthesis of Difluorobicyclo[1.1.1]pentanes
- Ellman's Sulfinamides
- The application of click chemistry in chemical ligation and peptide modification
- One of the "Golden Triangle" series of building blocks: acridine-based photoaffinity probes with "big" applications in a small body
- Innovations in the design of stereospecific drug molecular structures: Spirocyclic Scaffolds
- Spiralane: opening a new chapter of non-benzene pharmacy molecules
- Halogen Bond: Leading Drug Design into a New Chapter
- D-DTTA Salts of Azaindole Chiral Amines: New Options for Chemical Splitting
- Cyclic isomers--Azabicyclic molecular building blocks to aid drug design
- IACS-52825: A Potent and Selective DLK Inhibitor
- Action type of ligand acting on target
- Targeted drug discovery: scarce and inefficient, what is the geometry of its effectiveness?
- Explore the Industrialization Process of SOS1 Inhibitor MRTX0902
- The process path of EBL-3183: from indole to preclinical inhibitor
- Ellman's Sulfinamides
- Dextran: Multifunctional polysaccharides for biology and medicine
- read more
- Protocols
- Wolfe's Mineral Solution
- Alkaline Lysis Buffers A, B, C Recipes
- A Broth (Powder)
- Acrylamide (30%) Recipe
- Amies Broth with Charcoal (Amies Transport Medium) (Powder)
- Acrylamide (40%) Recipe
- 5-Fluoroorotic Acid Monohydrate (FOA, 5-FOA)
- Antibiotic Medium #1 (Powder)
- Bradford's Reagent Recipe
- Cell Culture Protocols
- Antibiotic Medium #3 (Powder)
- Antibiotic Medium #4 (Powder)
- Cell Staining Buffer Recipe
- Cell Stimulation Cocktail
- Antibiotic Medium #9 (Powder)
- Bromthymol Blue Recipe
- Antibiotic Medium #10 (Powder)
- CFSE Protocol
- Antibiotic Medium #11 (Powder)
- APT (All Purpose Tween) Agar (Powder)
- Azide Dextrose Broth (Powder)
- Bacillus cereus Medium (BCM) (Powder)
- Baird Parker Agar (Powder)
- BiGGY Agar (Powder)
- Bile Esculin Agar (Powder)
- Bismuth Sulfite Agar (Powder)
- Blood Agar Base No. 2 (Powder)
- Blood Agar Base, Low pH (Powder)
- Brain Heart Infusion Agar (Powder)
- Brain Heart Infusion Broth (Powder)
- Brain Heart Infusion Broth w/o Dextrose (Powder)
- Brilliant Green Agar (Powder)
- Brilliant Green Agar w/Sulfadiazine (Powder)
- Brilliant Green Bile Broth 2% (Powder)
- Bromthymol Blue
- Brucella Agar (Powder)
- Brucella Broth (Powder)
- Buffered Peptone Water (Powder)
- Campy Selective Agar Base (Powder)
- Casman Medium Base (Powder)
- Chapman Stone Medium (Powder)
- CLED Agar (Powder)
- CLED Agar, Bevis (Powder)
- Clostridium Difficile Agar (Powder)
- Clostrisel Agar (Powder)
- Columbia Blood Agar Base (Powder)
- Columbia CNA Agar (Powder)
- Cooked Meat Medium (Powder)
- Corn Meal Agar (Powder)
- Decarboxylase Broth Moeller (Powder)
- D/E Neutralizing Agar (Powder)
- Deoxycholate Agar (Powder)
- Deoxycholate Citrate Agar (Powder)
- Coomassie Blue Solution Recipe
- Denhardt Solution (50x) Recipe
- Deoxycholate Citrate Lactose Sucrose (DCLS) Agar (Powder)
- DEPC Treated Water Recipe
- Destain Solution Recipe
- DNA Loading Buffer (Orange G) Recipe
- DOT Blot Protocol
- ELISA Blocking Solution Recipe
- ELISA Coating Solution Recipe
- ELISA Methods
- Deoxycholate Lactose Agar (Powder)
- Dermatophyte Test Medium (DTM Test Agar) (Powder)
- Dextrose Agar (Powder)
- Dextrose Broth (Powder)
- ELISA Sample Collection & Storage
- Flow Cytometry General Protocol
- Glycerol Tolerant Gel Buffer 20X
- Immunocytochemistry
- Immunofluorescence General Protocol
- Immunohistochemistry General Protocols
- Dextrose Phosphate Broth (Powder)
- Dextrose Starch Agar (Powder)
- Immunoprecipitation General Protocol
- Dextrose Tryptone Agar (Powder)
- Dextrose Tryptone Broth (Powder)
- Deoxyribonuclease (DNase) Test Agar (Powder)
- Immunoprecipitation using Affinity Agarose Resins
- Deoxyribonuclease (DNase) Test Agar w/Toludine Blue (Powder)
- Laemmli Sample Buffer 2X
- Modified RIPA Buffer
- NADI Reagent
- Earle’s Balanced Salts (Powder)
- EC Medium (Powder)
- EC Medium w/MUG (Powder)
- NP-40 Cell Lysis Buffer
- Nuclease P1 from Penicillium Citrinum
- Elliker Broth (Powder)
- Pepsin
- Eosin Methylene Blue Agar (Powder)
- Phosphate Buffered Saline (PBS) 1x
- Eosin Methylene Blue Agar, Levine (Powder)
- Phosphate Buffered Saline (PBS) 10x
- Eugonic Broth (Powder)
- Phosphate Buffered Saline Tween-20 (PBST) 1x
- Red Blood Cell Lysing
- Red Blood Cell (RBC) Lysis Buffer
- RNA Extraction
- TAE 50x
- TBE 10x
- TE 1x
- TES
- Tissue Homogenization Buffer for ELISA
- TNE 1x
- Formaldehyde Gel Running Buffer
- Fluid Thioglycollate Medium w/K Agar (Powder)
- H Broth (Powder)
- L-Broth (Luria Broth) (Powder)
- Lambda Agar (Powder)
- Lambda Broth (Powder)
- LB Agar Lennox (Powder)
- Transfection Protocol
- LB Agar Lennox, Animal Free (Powder) (Lennox L agar)
- Tris Glycine Buffer 5x
- Tris-Buffered Saline (TBS)
- Tris-Buffered Saline Tween-20 (TBST)
- LB Agar Miller (Powder)
- TTE 1x
- Western Blot
- LB Agar Miller, Animal Free (Powder) (Miller's LB agar, Luria-Bertani agar)
- Western Blotting Transfer Buffer
- X-Gal Staining Solution
- Xylene Cyanol/Bromophenol Blue DNA Loading Buffer 10x
- Zymolyase
- LB Broth Lennox (Powder)
- LB Broth Lennox, Animal Free (Powder) (Lennox L broth base)
- LB Broth Miller (Powder)
- LB Broth Miller, Animal Free (Powder) (Miller's LB broth, Luria-Bertani broth)
- LPGA Agar Medium
- M63 Medium (Powder)
- M9 Minimal Salts (Powder)
- Nutrient Agar (Powder)
- Nutrient Agar 1.5% (Powder)
- Nutrient Broth (Powder)
- NZ Broth (Powder)
- NZC Broth (Powder)
- NZCYM Agar (Powder)
- NZM-Agar-Powder
- NZM Broth (Powder)
- NZYM Agar (Powder)
- NZYM Broth (Powder)
- SOB Agar (Powder)
- SOB Broth (Powder)
- SOC Broth (Powder)
- Sodium Citrate Buffer
- SSPE 20X
- STAB Agar (Powder)
- STET
- Super Broth (Powder)
- Terrific Broth (Powder)
- Terrific Broth, Complete with Carbon Source (Powder)
- Terrific Broth, Modified for Genomics (Powder)
- Terrific Broth, Modified for Fermentation, non-animal (Powder)
- Thermophilus Vitamin-Mineral Stock 100X (TYE Media, Castenholz Media) (Powder)
- Thermophilus Vitamin-Mineral Stock 1000X (TYE Media, Castenholz Media) (Powder)
- Tryptone Agar (Powder)
- Tryptone Broth (Powder)
- Two YT Broth (TY Broth) (Powder)
- Yeast Malt Extract Broth
- YT Broth (Powder)
- Synthetic Sea Water
- Synthetic Amino Acid Medium, Bacteriological, SAAM-B (Powder)
- Synthetic Amino Acid Medium, Fungal, SAAM-F (Powder)
- Culture Conditions and Types of Growth Media for Mammalian Cells
- Staining Dead Cells with Viability Dyes
- B5 Fixative Recipe
- Bouin's Fixative Recipe
- Ehrlich's Solution Recipe
- Field's Stain
- Flagella Stain
- Gentian Violet Stain
- Giemsa Stain
- Hellings 10X
- Intracellular Antigen Staining
- Kovac's Reagent
- Papanicolaou's Stain
- Paraformaldehyde (2%)
- Schiff's Reagent
- Tissue Fixation Solution
- Tissue Lysates Preparation
- Trypan Blue
- Zenker's Fixative
- PCR, Real-Time
- RT-PCR
- Hollande's Fixative
- Blood Agar Base, pH 7.4 (Powder)
- PCR, MIQE Guidelines
- Flow cytometry protocol
- Immunoprecipitation (IP) lysates and reagents
- read more
- Specifications, Grading and Purity
- EnzymoPure™: Excellence in Enzymatic Solutions for Biological and Chemical Industries
- Aladdin Scientific Product Grades Overview (the first one)
- Aladdin Scientific Product Grades Overview (the second one)
- ActiBioPure™: Premier Quality for Bioactive Recombinant Products
- UltraBio™: Defining Excellence in Molecular Biology Applications
- Difference between BioReagent and UltraBio™ Grades
- CellNourish™: High-Quality and Cost-Efficient Culture Media
- CellGuard Certified: Ensuring Excellence in Biological Applications
- Introducing Aladdin Scientific's Sub-Brands: Excellence in Every Product Line



