MCAT-53™ Catalyst for Ruthenium Formation
Product Manager:Nick Wilde

MCAT-53™, a novel ruthenium catalyst, simplifies C-H activated C-C coupling in water by eliminating extra steps and avoiding solvents like NMP.
Chicago Discovery Solutions has pioneered MCAT-53™, a recyclable and ligand-free ruthenium catalyst that enhances the synthesis of heterocyclic molecules through C-H activation and C-C bond formation in water, advancing sustainable catalytic functionalization reactions.
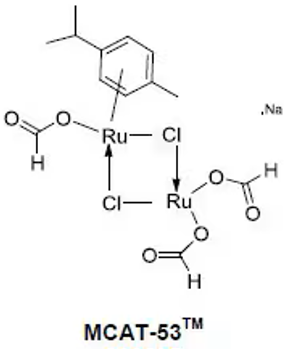
The Ruthenium Formato catalyst MCAT-53, exclusively developed by Chicago Discovery Solutions LLC, is ideally suited for promoting C-H activated C-C coupling reactions in an aqueous solution. This innovative catalyst stands out by eliminating the requirement for acids, co-solvents, surfactants, oxidants, ligands, and any additional activation procedures. By employing MCAT-53™, reactions become more eco-friendly, mitigating concerns over toxicity and disposal costs.2 Notably, upon completion and workup of the reaction, the aqueous medium can be efficiently repurposed (recycled) for subsequent catalytic reactions, promoting sustainability and cost-effectiveness.
Reactions catalyzed by MCAT-53™ effectively alleviate issues related to undesirable toxicity and disposal costs.2 Following the completion and processing of the reaction, the aqueous medium can be conveniently reused (recycled) for subsequent catalytic reactions, further enhancing its environmental and economic benefits.
Representative Application: synthesis of heterobiaryl compounds in water
Biaryl compounds are typically synthesized through established aryl-aryl coupling reactions. Traditional approaches for aryl-coupling and C-H bond activation, exemplified by the Suzuki-Miyaura and Stille cross-coupling reactions, involve intricate preparation of labile and hazardous organometallic reagents, with the reactions typically conducted in harsh organic solvents like DMF, NMP, DMSO, and toluene.1 In the modern era of catalytic cross-coupling, palladium-catalyzed systems, frequently requiring phosphine ligands, have emerged as the preeminent choice.

MCAT-53™ streamlines the synthesis process by eliminating the prerequisite step of converting the substrates' leaving groups (like halides) into substituted derivatives (e.g., boronic acids or esters).
This catalyst is uniquely designed to function efficiently in plain water, eliminating the need for acids, co-solvents, surfactants, or additional activation steps. Simply combining an aryl halide with an aromatic coupling partner in the presence of MCAT-53™ and a base (e.g. K2CO3) offers a safe and cost-effective alternative to expensive Pd-catalysts and precatalysts.3 Post-reaction workup is straightforward; if the product is solid, it may precipitate out and be easily filtered, thus avoiding the use of solvents for extraction after the reaction has been conducted in water.
A recent publication in Organic Research and Development, 2018, demonstrated the versatility of MCAT-53, showcasing its compatibility with a range of bromides, chlorides, and heavily substituted halides.5

Fig 1. Bromides chlorides structures
A diverse array of pyridine derivatives serves as outstanding directing groups, facilitating the synthesis of ortho-substituted products. Additionally, a broad spectrum of nitrogen-based directing groups, such as imines, oxime ethers, azobenzene derivatives, and nitrogen heterocycles (including pyrazoles and isoxazolines), can also be employed in these reactions.5 Furthermore, amides, with their inherently basic oxygen atoms, represent another viable option for directing these transformations.
Fig 2. Phenylpyrazol
- The C-H activated C-C coupling reactions have been successfully scaled up to 12 mmole scale using the MCAT-53 catalyst, demonstrating its effectiveness in facilitating these reactions.
- Remarkably, the catalyst loading for MCAT-53 has been reduced to just 1 mole % while still effectively carrying out C-H activated C-C coupling reactions.
- This scaled-up reaction, even with reduced catalyst loading, proves to be highly efficient as the product can be easily separated from the water layer through simple decanting.
- In cases where the product is solid, it may precipitate out directly and can be filtered off, eliminating the need for any additional solvents during the extraction process when water is used as the reaction solvent.
- The mono and di ratios in the reaction can be precisely manipulated, offering further control over the product distribution.
- When compared to other Ru-based catalysts, MCAT-53 stands out as significantly superior, yielding improved isolated product yields.
- After the reaction is complete, the spent aqueous layer can be recycled and reused, promoting sustainability and cost-effectiveness.
- The structural determination of MCAT-53 is based on rigorous analysis, providing a clear understanding of its composition and properties.
- MCAT-53 has been demonstrated to be effective in catalyzing reactions with a wide range of diverse substrates, including 2-phenyl pyridine, 2-phenyl pyrazole, and benzo(h)quinolone, showcasing its versatility and applicability.
Using the MCAT-53 catalyst, the synthesis of Anacetrapib, an advanced CETP inhibitor intermediate, yields a single regioisomer in water, demonstrating the catalyst's efficiency and selectivity.5
Fig 3. MCAT-53 catalyst
First-in-Class Catalyst: MCAT-53, the first of its kind, combines bench and air stability, making it an attractive choice for cost-effective and environmentally sustainable alternatives in pharmaceutical and diverse chemical manufacturing processes.
Commercial Accessibility & Versatility: The commercial availability of MCAT-53, coupled with its air stability and convenient handling, presents a promising opportunity for its application in numerous other chemical transformations, broadening its utility across various industries.
References
1. Lyons TW, Sanford MS. 2010. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev.. 110(2):1147-1169. https://doi.org/10.1021/cr900184e
2. Arockiam PB, Bruneau C, Dixneuf PH. 2012. Ruthenium(II)-Catalyzed C-H Bond Activation and Functionalization. Chem. Rev.. 112(11):5879-5918. https://doi.org/10.1021/cr300153j
3. Davies HML, Morton D. 2016. Recent Advances in C-H Functionalization. J. Org. Chem.. 81(2):343-350. https://doi.org/10.1021/acs.joc.5b02818
4. 2016. Ali Aiden KOOHANGAnita Mehta. Ruthenium-catalyzed synthesis of biaryl compounds in water. US20160263565A1
5. Mehta A, Saha B, Koohang AA, Chorghade MS. 2018. Arene Ruthenium Catalyst MCAT-53 for the Synthesis of Heterobiaryl Compounds in Water through Aromatic C-H Bond Activation. Org. Process Res. Dev.. 22(9):1119-1130. https://doi.org/10.1021/acs.oprd.8b00141
Aladdin:https://www.aladdinsci.com


