Catecholborane
Sandra Forbes
Product Manager
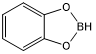
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.
Recent Literature
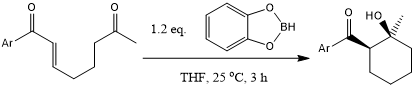
A tandem 1,4-reduction-aldol cyclization is induced by exposure of monoenone monoketones to catecholborane in THF at ambient temperature. Six-membered cyclic aldol products are formed in excellent yield with high levels of syn diastereoselectivity for aromatic and heteroaromatic enones. Five-membered ring formation proceeds less readily, but the yield is improved through addition of Rh(I) salts.
R. R. Huddleston, D. F. Cauble, M. J. Krische, J. Org. Chem., 2003, 68, 11-14.
DOI: 10.1021/jo020629f
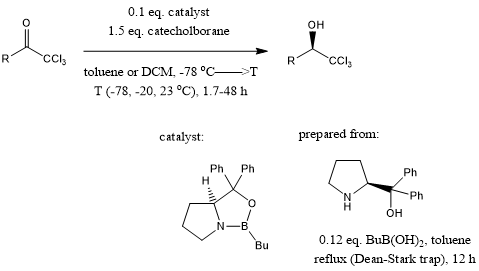
A novel enantioselective synthesis of α-amino acids has been developed, which is broad in scope, simple in application, and advantageous for many α-amino acids of interest in chemistry, biology, medicine.
E. J. Corey, J. O. Link, J. Am. Chem. Soc., 1992, 114, 1906-1908.
DOI: 10.1021/ja00031a069
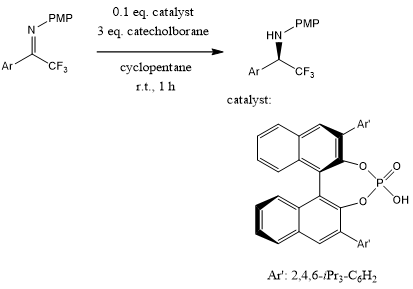
A BINOL-derived boro-phosphate catalyzes an enantioselective reduction of α-trifluoromethylated imines to provide chiral α-trifluoromethylated amines in high yields and with excellent enantioselectivities in the presence of catecholborane as hydride source under mild conditions.
H. He, X. Tang, Y. Cao, J. C. Antilla, J. Org. Chem., 2021, 86, 4336-4345.
Hydroboration with catecholborane, followed by treatment with easily available reagents such as alkenyl sulfones or alkynyl phenyl sulfones in the presence of a radical initiator, represents an effective and simple one-pot procedure for direct vinylation, formylation, and cyanation.
A.-P. Schaffner, V. Darmency, P. Renaud, Angew. Chem. Int. Ed., 2006, 45, 5847-5849.
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/catecholborane.shtm
Aladdinsci: https://www.aladdinsci.com/

