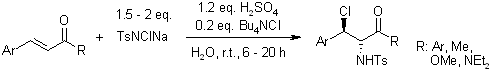Chloramine-T, N-chloro tosylamide sodium salt

Product Manager:Nick Wilde
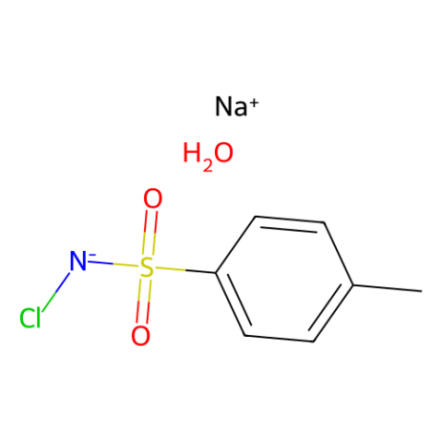
Chloramine-T is a source of electrophilic chlorine. In water, chloramine-T is decomposed to yield hypochlorite, which acts as a disinfectant, and the sulfonamide moiety, which inhibits bacterial grow due to the similarity with para-aminobenzoic acid (a bacterial metabolite). Chloramine-T can therefore be used as a biocide and a mild disinfectant.
Recent Literature
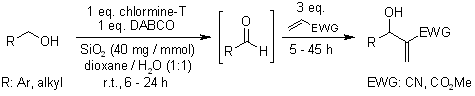
Silica gel-DABCO catalyzed oxidation of alcohols to aldehydes with chloramine-T followed by their Morita-Baylis-Hillman reaction with acrylonitrile or methyl acrylate gives good overall yields of the corresponding Morita-Baylis-Hillman adducts. The present work opens up a new and efficient synthetic route to Morita-Baylis-Hillman adducts directly from alcohols in a one-pot operation.
L. D. S. Yadav, V. P. Srivasta, R. Patel, Synlett, 2010, 1047-1050.
https://doi.org/10.1055/s-0029-1219577
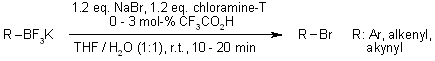
Organotrifluoroborates are rapidly and regioselectively converted into organic bromides in excellent yields under mild conditions, using sodium bromide in the presence of chloramine-T.
G. W. Kabalka, A. R. Mereddy, Organometallics, 2004, 23, 4519-4521.
https://doi.org/10.1021/om049685s
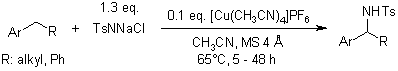
Benzylic hydrocarbons are selectively converted to the corresponding sulfonamides by the [Cu(CH3CN)4]PF6-catalyzed reaction with anhydrous TolSO2NNaCl (chloramine-T). Under the same conditions, representative ethers and olefins are also amidated.
R. Bhuyan, K. M. Nicholas, Org. Lett., 2007, 9, 3957-3959.
https://doi.org/10.1021/ol701544z
A practical, regio- and diastereoselective synthesis of vicinal chloramines from electron-deficient olefins and Chloramine-T is promoted by Brønsted acids in water. This novel protocol is efficient, mild, ecofriendly, and broadly applicable for the aminochlorination of various electron-deficient olefins including α,β-unsaturated ketones, cinnamate, and cinnamide.
X.-L. Wu, G.-W. Wang, J. Org. Chem., 2007, 72, 9398-9401.
https://doi.org/10.1021/jo701957t
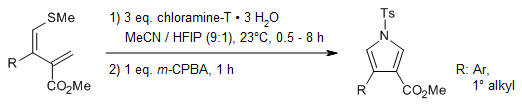
Ring-opening of readily available 2,5-dihydrothiophenes provides access to a panel of 1,3-dienes which undergo pyrrole formation in the presence of inexpensive chloramine-T trihydrate. The transformation is conducted in an open flask and proceeds at ambient temperatures.
F.-L. Haut, N. J. Feichtinger, I. Plangger, L. A. Wein, M. Müller, T.-N. Streit, K. Wurst, M. Podewitz, T. Magauer, J. Am. Chem. Soc., 2021, 143, 9002-9008.
https://doi.org/10.1021/jacs.1c04835
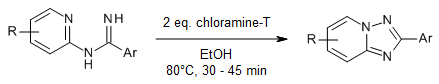
A convenient synthesis of 1,5-fused 1,2,4-triazoles in high yields from readily available N-arylamidines is efficiently promoted by chloramine-T through direct metal-free oxidative N-N bond formation. The mild nature of the synthesis and short reaction time are notable advantages of the developed protocol.
A. S. Singh, A. K. Agrahari, S. K. Singh, M. S. Yadav, V. K. Tiwari, Synthesis, 2019, 51, 3443-3450.
https://doi.org/10.1055/s-0037-1611906
Quoted
For more information, visit our website: www.aladdinsci.com.