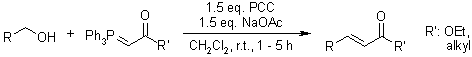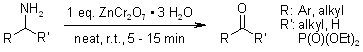Chromium Compounds

Product Manager:Nick Wilde
Chromium (VI) compounds pose a significant health risk due to their high toxicity, both acutely and chronically, with the potential to cause cancer. In contrast, chromium (III) is vital for human health and exhibits moderate toxicity. When handling chromium (VI) reagents, such as PDC, in their finely powdered form, extreme caution is necessary to prevent inhalation or oral exposure, as these compounds are extremely toxic.
In cases where chromium compounds are used excessively, often for the oxidation of alcohols to carbonyl compounds and carboxylic acids, any residual chromium (VI) can be effectively neutralized by adding 2-propanol. This process is indicated by a noticeable color change from an orange hue to a deep green, signifying complete quenching.
See also: Chromium Trioxide, Collins Reagent, Jones Reagent, Sarett Reagent, Pyridinium Chlorochromate, Pyridinium Dichromate
A list of alternative and somewhat more environmentally friendly oxidants for the oxidation of alcohols is given here for the preparations of aldehydes, ketones and carboxylic acids.
A full review of chromium-based reagents can be found in the book written by Tojo and Fernández (Oxidation of Alcohols to Aldehydes and Ketones, Springer Berlin, 2006, 1-97.).
Recent Literature
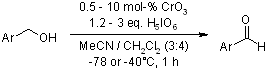
Benzyl alcohols and benzyl TBDMS ethers underwent efficient oxidation to their respective carbonyl compounds, achieving high yields under mild conditions using periodic acid catalyzed by CrO3 at a temperature of -78 °C. This oxidation process demonstrated remarkable functional group tolerance and exceptional selectivity towards the TBDMS group compared to the TBDPS group, making it an effective method for the targeted transformation of these compounds.
S. Zhang, L. Xu, M. L. Trudell, Synthesis, 2005, 1757-1760.
https://doi.org/10.1055/s-2005-869975

S. Zhang, L. Xu, M. L. Trudell, Synthesis, 2005, 1757-1760.
https://doi.org/10.1055/s-2005-869975

The CrO3-catalyzed oxidation of primary alcohols to carboxylic acids proceeds smoothly and efficiently, requiring only 1-2 mol% of CrO3 and 2.5 equivalents of H5IO6 in a wet MeCN solution. This process yields carboxylic acids in excellent purity, without significant racemization occurring for alcohols that possess adjacent chiral centers. Furthermore, secondary alcohols undergo clean oxidation to ketones, highlighting the versatility of this catalytic system.
M. Zhao, J. Li, Z. Song, R. Desmond, D. M. Tschaen, E. J. J. Grabowski, P. J. Reider, Tetrahedron Lett., 1998, 39, 5323-5326.
https://doi.org/10.1016/S0040-4039(98)00987-3
A straightforward and quantitative method for the preparation of carboxylic acids involves the oxidation of primary alcohols and aldehydes using pyridinium chlorochromate (PCC) as a catalyst at a concentration of 2 mol%. This process utilizes 2.2 equivalents of H5IO6 for the oxidation of primary alcohols and 1.1 equivalents for aldehydes, both in an acetonitrile solvent. The resulting carboxylic acids are obtained in high yield, demonstrating the effectiveness of this PCC-catalyzed oxidation approach.
M. Hunsen, Synthesis, 2005, 2487-2490.
https://doi.org/10.1055/s-2005-872085
M. Hunsen, Synthesis, 2005, 2487-2490.
https://doi.org/10.1055/s-2005-872085
A domino oxidation process utilizing a combination of pyridinium chlorochromate (PCC) and sodium acetate (NaOAc), in conjunction with stabilized Wittig reagents, enables the efficient conversion of primary alcohols into α,β-unsaturated compounds. This method offers a streamlined approach to the synthesis of these valuable chemical intermediates.
J. Shet, V. Desai, S. Tilve, Synthesis, 2004, 1859-1863.
https://doi.org/10.1055/s-2004-829123
A rapid and efficient oxidative deamination reaction, facilitated by ZnCr2O7•3H2O under solvent-free conditions at room temperature, enables the synthesis of α-ketophosphonates from various α-aminophosphonates. This methodology also demonstrates its versatility in the rapid and highly selective oxidation of a wide range of amines to aldehydes and ketones, achieving excellent yields.
S. Sobhani, M. F. Maleki, Synlett, 2010, 382-386.
https://doi.org/10.1055/s-0029-1219174
Quoted
from:https://www.organic-chemistry.org/chemicals/oxidations/chromium-compounds.shtm
For more information, visit our website: www.aladdinsci.com.