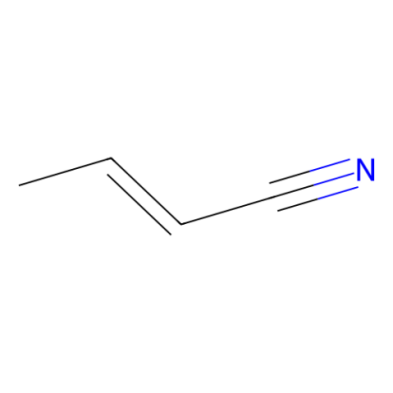
Crotononitrile, (E)-But-2-enenitrile
Product Manager:Nick Wilde
Crotononitrile is a good hydrogen acceptor in ruthenium-catalyzed hydrogen transfer reactions.
Recent Literature
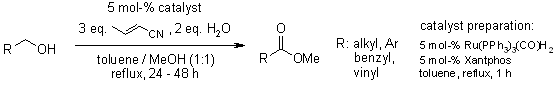
Alcohols and aldehydes can be oxidized to the corresponding methyl esters by reaction with methanol in the presence of crotononitrile as a hydrogen acceptor using a catalyst combination of Ru(PPh3)3(CO)H2 with xantphos.
N. A. Owston, T. D. Nixon, A. J. Parker, M. K. Whittlesey, J. M. J. Williams, Synthesis, 2009, 1459-1462.
https://doi.org/10.1055/s-0028-1088026

N. A. Owston, T. D. Nixon, A. J. Parker, M. K. Whittlesey, J. M. J. Williams, Synthesis, 2009, 1459-1462.
https://doi.org/10.1055/s-0028-1088026
Ruthenium-catalyzed hydrogen transfer of 1,3-diols in the presence of alkyl hydrazines provides 1,4-disubstituted pyrazoles. A regioselective synthesis of unsymmetrical pyrazoles from β-hydroxy ketones can also be achieved.
D. C. Schmitt, A. P. Taylor, A. C. Flick, R. E. Kyne, Jr., Org. Lett., 2015, 17, 1405-1408.
https://doi.org/10.1021/acs.orglett.5b00266
In a new version of the Fischer indole synthesis, primary and secondary alcohols have been catalytically oxidized in the presence of phenylhydrazines and Lewis acids to give the corresponding indoles in one step. The use of alcohols instead of aldehydes or ketones broadens the scope of available starting materials and offers easy handling and safety.
A. Porcheddu, M. G. Mura, L. De Luca, M. Pizzetti, M. Taddei, Org. Lett., 2012, 14, 6112-6115.
https://doi.org/10.1021/ol3030956
Quoted from:https://www.organic-chemistry.org/chemicals/oxidations/crotononitrile.shtm
Aladdin:https://www.aladdinsci.com


