Determination of Additives in Beverages
INTRODUCTION

Beverages, such as common sodas and energy drinks, may contain many polar ingredients that are easily dissolved in the aqueous matrix of the beverage. These ingredients include sweeteners (sugar or sugar substitutes), caffeine, vitamin supplements, amino acids, organic acids, and plant extracts. Because the sample to be analyzed is already in solution, no additional sample preparation process is required, but the solution can be diluted and injected directly into high performance liquid chromatography (HPLC).
In this technical article, we introduce the application of two kinds of fast high performance liquid chromatography columns for beverages. One advantage of these columns, which are suitable for a wide range of HPLC systems, is that they can achieve the resolution, efficiency, and speed associated with the use of sub2 micron columns on UHPLC systems without high back pressure. Chemical columns (RP-amide and HILIC) have been selected for this technical article because they have stronger polar compound characteristics compared to C18.
ANALYSIS OF DIET SODA
Artificial sweeteners are often used in drinks in specific combinations to mimic the sweetness of natural sugars. They contain less energy than sugar and are added to maintain taste without adding extra calories. In addition to sweeteners, sodas may also contain preservatives, which are added to inhibit microbial growth to extend the shelf life of the drink. Because artificial sweeteners and preservatives are considered additives, they are often regulated; Therefore, it is necessary to determine their properties and add concentrations.
Figure 1 shows the HPLC separation of three artificial sweeteners (acesulfamil, aspartame, and neotame) and two preservatives (benzoic acid and sorbate) caffeine. In this example, a fast RP-amide column is used. The amide functional group in the amide column provides a selectivity different from the pure phase of the alkyl group (such as C18). The difference in selectivity is most pronounced for polar compounds, such as those in this application. On the fast RP-amide column, the separation of all major components can be achieved in less than 1 minute using a conventional HPLC system that accepts back pressure (3200 psi/220 bar).
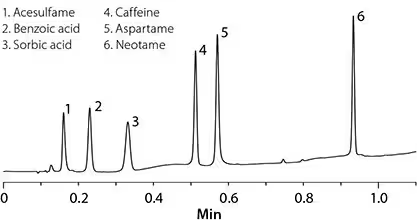
Figure 1. Analysis of artificial sweeteners, preservatives and caffeine in diet soda by high performance liquid chromatography
HPLC Conditions
Chromatographic column: Rapid RP-amide column, 3 cm x 4.6 mm I.D., 2.7 µm diameter;
Mobile phase: (A) 100 mM ammonium acetate solution, pH = 5.6, with acetic acid titration to calibrate the concentration; (B) Water; (C) acetonitrile;
Gradient: 20% constant speed A, C from 5 to 60% C in 1 minute, and maintain 60% C for 0.1 minutes;
Flow rate: 3 mL/ min;
Column temperature: 40 °C;
Detector: UV at 214 nm;
Injection capacity: 1µL;
Sample: Diet soda in a buffer solution at 100-500 µg/mL.
ANALYSIS OF CAFFEINATED ENERGY DRINK
Energy drinks contain a variety of marketable components, such as sugars, vitamins, and caffeine, and like soda, they often contain preservatives, as shown in Figure 2 for an analysis of energy drinks on a rapid HILIC column. HILIC chromatography was chosen because it is suitable for the retention and separation of hydrophilic compounds based on polarity differences, whether these analytes are acidic, alkaline, charged, or neutral. Different types of compounds can be detected by ultraviolet spectrophotometry and ELSD method. ELSD can detect sugars that do not absorb ultraviolet light.
The analysis was performed using an MS-friendly mobile phase with fast analysis speed (less than 2 minutes) and low back pressure (815 psi). Because of these advantages, HILIC columns can be used to analyze beverage components.
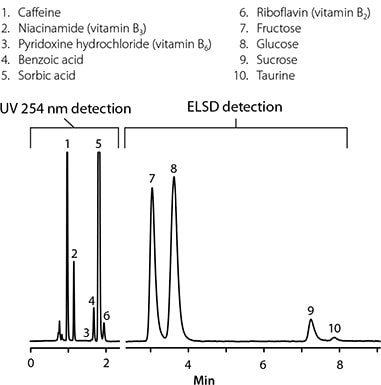
Figure 2. HPLC analysis of sugars, vitamins, preservatives and caffeine in energy drinks
HPLC Conditions
Chromatographic column: Rapid HILIC column, 10 cm x 3.0 mm I.D., 2.7 µm diameter;
Mobile phase: (A) 100 mM ammonium acetate solution, pH = 5.0; (B) Water; (C) acetonitrile; (A: B: C = 9:1:90);
Flow rate: 0.6mL/min;
Column pressure: 815 psi;
Column temperature: 35 °C;
Detector: UV or ELSD at 254 nm, 55 °C, 3.5 bar nitrogen;
Injection capacity: 2µL;
Sample: Commercial energy drink diluted with acetonitrile 1:9.
CONCLUSION
These simple examples demonstrate the application of two rapid columns in beverage analysis, both of which are suitable for the analysis of polar compounds and can be successfully used to analyze several beverage components, including sugars, vitamins, sweeteners, preservatives and caffeine. The fast column provides a fast separation method for traditional HPLC systems without the significant increase in back pressure associated with sub2 micron columns. The selectivity difference between the two columns can be further used to change the elution order and compound retention time.
