Determination of chloride and sulfate in saturated lithium hydroxide solution
Sandra Forbes
Product Manager

Goal
To demonstrate the determination of chloride and sulfate in saturated lithium hydroxide solution.
Introduction
Lithium hydroxide is an important compound for industry. It is used in many applications, such as battery manufacturing (lithium-ion and lithium polymer batteries), welding, electroplating painting (pigments, binders, and biocides), cleaning products, and household care.
Rechargeable batteries are an increasing part of our daily life as we use more portable electronic devices, including mobile phones. These batteries are also important for the electric car industry. Lithium-ion batteries are the most commonly used rechargeable batteries because of their high volumetric energy density. Lithium hydroxide and lithium carbonate are precursors to make lithium compounds used in lithium-ion batteries. Determination of chloride and sulfate in saturated lithium hydroxide and lithium carbonate solutions, and using those values to determine the amounts in the solid, is desired by both battery recyclers and chemical suppliers.
A method was developed for lithium carbonate. This application note demonstrates the determination of chloride and sulfate in saturated lithium hydroxide solution using a Thermo Scientific™ Dionex™ Reagent-Free Ion Chromatography™ (RFIC™) system with a Thermo Scientific™ Dionex™ IonPac™ AS29-Fast-4µm anion-exchange column. The method includes sample preparation using Thermo Scientific™ Dionex™ OnGuard™ II H cartridges.
Experimental
Equipment and consumables
• A Dionex ICS-5000+ Ion Chromatography (RFIC) system* was used in this work, which includes:
– Dionex ICS-5000+ SP/DP Pump module
– Dionex ICS-5000+ EG Eluent Generator module with high-pressure degasser module
– Dionex ICS-5000+ DC Detector/Chromatography module with conductivity detector and dual temperature zones
• Thermo Scientific™ Dionex™ AS-AP Autosampler with 250 µL syringe, 1.2 mL buffer line assembly, and 5.0 µL injection loop
• Thermo Scientific™ Chromeleon™ 7.2 Chromatography Workstation
Consumables
• Thermo Scientific™ Dionex™ EGC 500 K2CO3 Potassium Carbonate Eluent Generator Cartridge (EGC)
• Thermo Scientific™ Dionex™ EPM 500 Electrolytic pH Modifier
• Thermo Scientific™ Dionex™ EGC Carbonate Mixer Kit, 2 mm
• Thermo Scientific™ Dionex™ AERS™ 500 Carbonate electrolytically regenerated suppressor (2 mm)
• Thermo Scientific™ Dionex™ IonPac™ AG29-Fast-4µm, Guard Column (2 × 30 mm)
• Thermo Scientific™ Dionex™ IonPac™ AS29-Fast-4µm, Analytical Column (2 × 150 mm)
• Thermo Scientific™ Dionex™ OnGuard™ II H cartridges, 1 mL
• Thermo Scientific™ Nalgene™ Syringe filter (0.2 μm, PES membrane)
• Thermo Scientific™ Dionex™ AS-AP Autosampler vials. Package of 100, 10 mL polystyrene vials with caps and blue septa
System preparation and setup
Figure 1 shows the flow diagram of the IC system used for the determination of chloride and sulfate in lithium hydroxide solution. A high-capacity anion-exchange column, the Dionex IonPac AS29-Fast-4µm column, was used. This column was selected because it was designed for fast determinations of inorganic anions in a variety of challenging samples, including high pH, low pH, and high ionic strength samples using carbonate/bicarbonate eluents. The IC system is plumbed as an RFIC system using eluent generation following the Dionex Eluent Generator Cartridges product manual. The suppressor is installed in recycle mode.
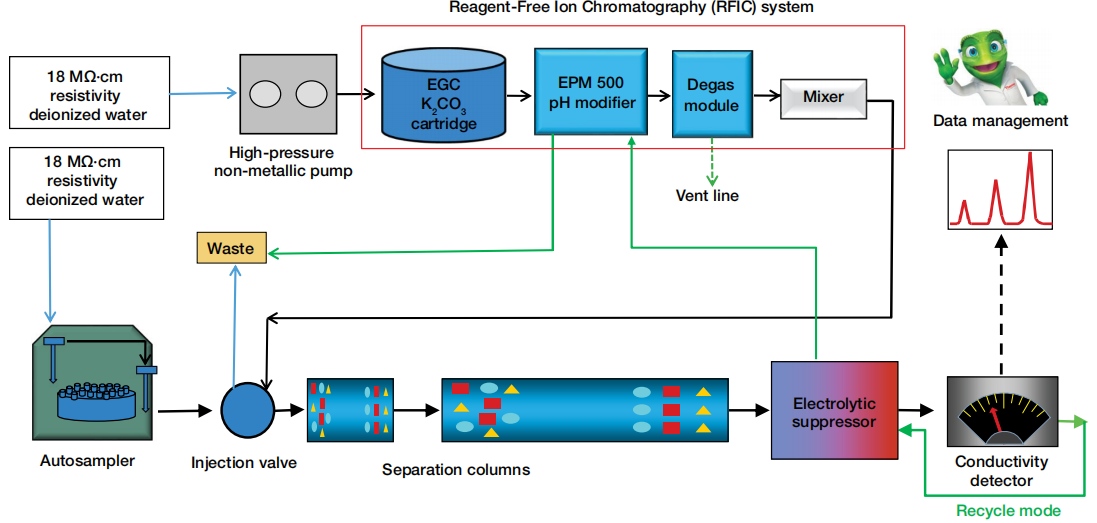
Figure 1. Illustration of the IC system flow diagram
Chromatography conditions
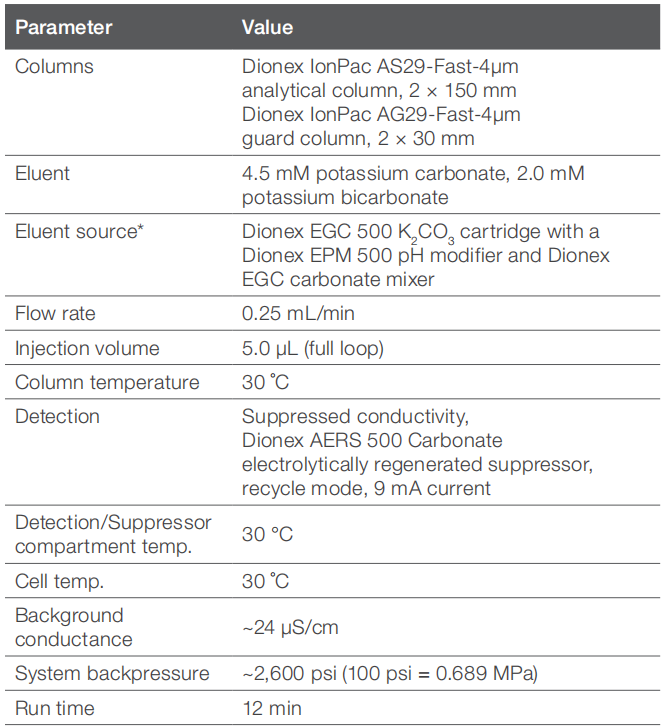
Reagent and standards
• Degassed deionized (DI) water, 18 MΩ·cm resistance or better
• Lithium hydroxide, Monohydrate (Powder, ACS Certified)
• Sodium chloride (ACS, ≥99%, Ultrapure)
• Sodium sulfate (99+%, for HPLC, anhydrous)
Preparation of solutions and reagents
Stock standard solutions (1,000 mg/L)
Stock standard solutions were prepared by dissolving the appropriate amounts of the required analytes in 100 mL of DI water, according to Table 1. The 1,000 mg/L standard solutions are also available. Stock standards were stored at 4 ˚C.

Table 1. Masses of compounds used to prepare 100 mL of 1,000 mg/L anion standards
Calibration standard solutions
Calibration standards were prepared by diluting the 1,000 mg/L stock standards with DI water (Table 2).
Table 2. Calibration standards (mg/L)
Method detection limit (MDL) standards
The mixed standards containing low concentrations of chloride and sulfate were prepared for their estimated MDLs. The MDLs were determined using a mixed standard containing 0.01 mg/L chloride and 0.01 mg/L sulfate.
Sample preparation
Saturated lithium hydroxide
Saturated lithium hydroxide sample was prepared by adding excess lithium hydroxide monohydrate solid to DI water (~10 g/50 mL) and allowing it to dissolve overnight to reach saturation. The solubility of lithium hydroxide in water is 12.8 g/100 mL at 20 °C.
Lithium hydroxide sample
Saturated lithium hydroxide cannot be analyzed directly. It must be diluted before analysis. In-line sample preparation using a Thermo Scientific™ Dionex™ Guardcap™ H vial cap did not work due to the capacity of the cap. Here, we used a Dionex OnGuard II H cartridge as follows.
1. Before application of the sample, slowly (<2 mL/min) flush the Dionex OnGuard II H cartridge first with 10 to 15 mL of deionized (DI) water using a 5 mL syringe.
2. Push 5 to 10 mL of air to remove the DI water from the cartridge.
3. Prepare 10x diluted sample by mixing 1 part saturated lithium hydroxide with 9 parts DI water.
4. Push 4 mL of the 10x diluted sample from step 3 slowly (<2 mL/min) through the previously prepared Dionex OnGuard II H cartridge. Discard the first 2 mL. Collect the second 2 mL fraction for analysis.
Spiked samples
To evaluate method accuracy, two spiked samples were prepared by adding a known amount of mixed chloride and sulfate standard into the saturated lithium hydroxide solution. The spiked samples were also prepared through steps 1 to 4 above before analysis.
Results and discussion
Separation
The Dionex IonPac AS29-Fast-4µm column is a high-capacity anion-exchange column designed for the determination of anions, including chloride and sulfate, in aqueous samples using suppressed conductivity detection. It can be used in a variety of sample matrices including high pH, low pH, and high ionic strength matrices. The column is designed to be used with an isocratic carbonate/bicarbonate eluent, which can be prepared from concentrated eluent stock or produced by an electrolytic eluent generator.
Figure 2 shows a separation of chloride and sulfate within 12 min using a Dionex IonPac AS29-Fast-4µm column. Chloride and sulfate are well resolved. A carbonate dip at ~6 min is observed. The carbonate can appear as a dip or a peak depending on the relative carbonate concentration in the sample compared to the eluent. The dip observed here indicates that the carbonate concentration in the sample is lower than in the eluent. One of the advantages for the Dionex IonPac AS29-Fast-4µm column is that carbonate is well separated from all common anions, including chloride and sulfate.
Figure 2. Chromatograms of a mixed standard and a lithium hydroxide sample
Using manually prepared eluent, the anion impurities in the concentrated eluent stock will cause interference, a dip or peak at the retention time of the impurity. To avoid the interferences caused by chloride and sulfate impurities in the eluent, a RFIC system to generate high purity eluent is recommended for this application.
Linearity and method detection limits (MDLs)
The six-point calibrations for chloride and sulfate were evaluated (Table 2). MDLs were determined by performing seven replicate injections of standards at a concentration less than ten times the estimated MDLs. The MDLs for saturated lithium hydroxide were determined by multiplying the method MDLs by ten.
Table 3 shows the retention times, linear concentration ranges, the coefficients of determination (r2), and calculated MDLs for chloride and sulfate. The method shows linear relationships (Figure 3) of peak area to concentration in the chosen calibration ranges with coefficients of determination (r2) of 0.9992 for chloride and 0.9999 for sulfate. The method is sensitive for the determination of chloride and sulfate in saturated lithium hydroxide with low MDLs (0.09 mg/L for chloride and 0.13 mg/L for sulfate).
Table 3. Linearity, method detection limit (MDL), and retention time obtained using a Dionex IonPac AS29-Fast-4µm column with a 5.0 µL injection
Chloride and sulfate in lithium hydroxide
Chloride and sulfate were determined in the saturated lithium hydroxide solution (Figure 2 and Table 4). This saturated lithium hydroxide solution contains 1.1 mg/L chloride and 3.2 mg/L sulfate.
Method precision and accuracy
The method precision and accuracy were evaluated by spiking chloride and sulfate in the saturated lithium hydroxide solution (Table 5). The method is precise (RSD range 1–6%) and accurate (recovery range 91–102%).
Figure 3. Calibration plots
Table 4. Chloride and sulfate in the saturated lithium hydroxide solution
Table 5. Spike recovery of chloride and sulfate in saturated lithium hydroxide
Conclusion
This application note describes a method for the determination of chloride and sulfate in saturated lithium hydroxide solution using an RFIC system with a 2 mm version of a Dionex IonPac AS29-Fast-4µm column. The method is sensitive (MDL = 0.09 mg/L for chloride and 0.13 mg/L for sulfate in saturated lithium hydroxide solution), precise (RSD range = 1–6%), and accurate (recovery range = 91–102%).
References
1. Lithium hydroxide. https://pubchem.ncbi.nlm.nih.gov/compound/Lithium-hydroxide
2. Qi Li, et.al. Review article: Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy & Environment 2016, 1(1), 18–42. https://doi.org/10.1016/j.gee.2016.04.006
3. Thermo Scientific Application Note 001967: Determination of Inorganic Anions in Saturated Lithium Carbonate Solution, Sunnyvale, CA, USA, 2023. https://appslab.thermofisher.com/App/4822/determination-anions-lithium-carbonate
4. Thermo Scientific Dionex ICS-5000+ Ion Chromatography System Operator's Manual. https://assets.thermofisher.com/TFS-Assets/CMD/manuals/man-065446-ics-5000-plus-man065446-en.pdf
5. Thermo Scientific Dionex IonPac AS29-Fast-4µm anion-exchange column. https://www.thermofisher.com/order/catalog/product/302833
6. Thermo Scientific Dionex OnGuard II H Cartridges. https://www.thermofisher.com/order/catalog/product/082761
7. Thermo Scientific Dionex Eluent Generator Cartridges-Product Manual. https://www.thermofisher.com/order/catalog/product/088453#/088453
8. Thermo Scientific Dionex AERS 500 Carbonate Electrolytically Regenerated Suppressor (2 mm). https://www.thermofisher.com/order/catalog/product/085028
9. Thermo Scientific Application Update 72331: In-line Sample Preparation for the Determination of Anions in Sodium Hydroxide, Sunnyvale, CA, USA, 2017. https://appslab.thermofisher.com/App/3856/au72331-inline-sample-preparation-for-determination-anions-sodium-hydroxide
Aladdinsci: https://www.aladdinsci.com/







