Dextran Chemistry
Dextran Chemistry
The Dextran Chemistry section presents a brief summary of the physical and chemical properties of Dextran. Furthermore, the section gives a short overview of common applications of Dextran.
The information presented here are based data from literature and in-house knowledge.
Dextran Properties:
Dextran Characteristics
Dextran fractions are characterized by their average molecular weights and molecular weight distributions.
Dextran is used in various fields such as pharmaceutical, photographic, and agricultural industries.
The versatile use of Dextran products relates to the favorable properties:
Dextran is neutral and water soluble.
Dextran is easily filtered.
Dextran is biocompatible.
Dextran is biodegradable.
Dextran is stable for more than 5 years.
Dextran Structure
Dextran is an α-D-1,6-glucose-linked glucan with side-chains 1-3 linked to the backbone units of the Dextran biopolymer. The degree of branching is approximately 5% in native (unfractionated) dextran and decreases with decreasing molecular weight. The branches are mostly 1-2 glucose units long.
Dextran can be obtained from fermentation of sucrose-containing media by Leuconostoc mesenteroides B512F.
A fragment of the Dextran structure is illustrated in Figure 1.
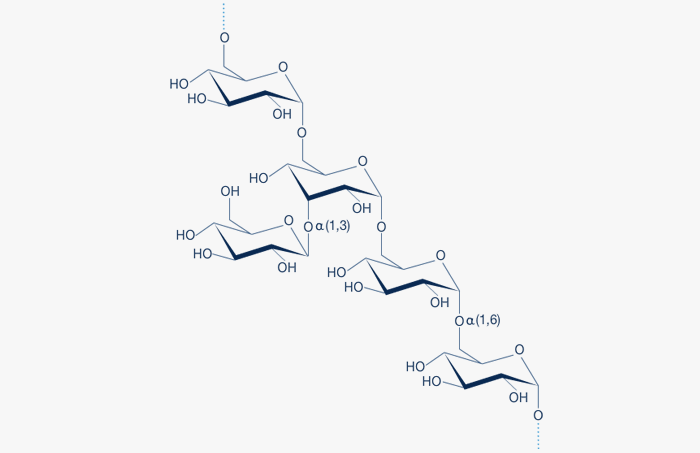
Fig. 1: Dextran is a glucose polymer in which the linkages are predominantly of the α(1,6) type. The degree of α(1,3) branching is generally less than 5% and decreases with decreasing molecular weight
Physical Properties of Dextran
Molecular Weight and Size
Dextran fractions are supplied in molecular weights from 1,000 Daltons to 2,000,000 Daltons (1). The molecular weight of the fraction is in most cases a key property and is defined in terms of the average molecular weight Mw (2) and the number average molecular weight Mn (3).
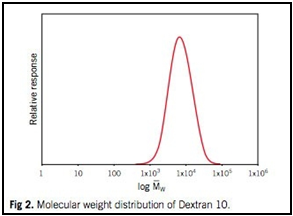
The molecular weight distribution curve for each fraction obtained by gel chromatography offers a unique method for characterizing the Dextran fraction.
Molecular weight distribution of Dextran 10 is illustrated in Figure 2.
Dextran Molecular Weight
The designation 5, 10, etc. represents the mean molecular weight divided by 1,000 Daltons. Thus Dextran 10 corresponds to a mean molecular weight of 10,000 Daltons.
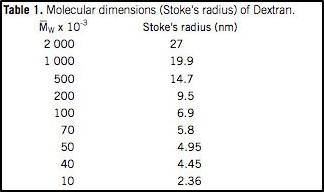
Dextran fractions behave as very flexible and extended polymers and in solution exist as an expandable coil. The molecular dimensions of some Dextran fractions are shown in Table 1.
Molecular Dimensions Of Dextran
Solubility of Dextran Fractions
Dextran fractions are readily soluble in water and electrolyte solutions to form clear, stable solutions. The pH does not affect solubility significantly. Concentrated solutions (> 50% w/v) may be prepared.
Dextran fractions are also soluble in some other solvents, notably, methyl sulfide, formamide, ethylene glycol, and glycerol. Dextran fractions are insoluble in monohydric alcohols, for example methanol, ethanol and isopropanol, and also most ketones, such as acetone and 2-propanone.
Although Dextran fractions will form clear solutions, it should be noted that the lowest molecular weight fractions 5 and 10 may, on standing, form turbid solutions, particularly when concentrated solutions are used. This effect may be delayed by boiling the solutions immediately after preparation.
Filtration of Dextran Solutions
Dextran fraction solutions can be filtered without difficulty. More concentrated solutions will require larger filters or filter series and higher pressures in order to increase the rate of filtration. Further increases in filtration rates may be achieved by raising the temperature. The dimensions of the filter system must be related to the volume and concentration of the Dextran solution used.
Viscosity of Dextran Solutions
Dextran fraction solutions exhibit Newtonian flow characteristics, that is, the flow rate is independent of shear stress.
Figure 3 shows the dependence of viscosity on concentration for Dextran fractions at 25 °C.

As Dextran is a neutral polysaccharide, the viscosity is not significantly influenced by changes in pH or salt concentration.
Colloid Osmotic Pressure of Dextran Solutions
The colloid osmotic pressure is important for many applications using Dextran. When comparing osmotic pressures, it is important that the molecules do not pass through the membrane with which they are in contact. For similar solute concentrations, osmotic pressure will be largely dependent on the molecular weight of the solute. Since Dextran is a neutral polymer with large dimensions, it will not easily permeate many human tissues and will thus maintain a favorable osmotic environment unlike, for example, saline which readily diffuses into cells and tissues.
A comparison of colloid osmotic pressures for Dextran fractions 40 and 70 is shown in Figure 4.

Specific Optical Rotation
[α]D = +195 – +201 (at 25 °C)
where [α] is the specific optical rotation measured in the sodium D line.
Below approximately 20,000 Daltons the specific optical rotation decreases with decreasing molecular weight.
Da : When nothing is indicated molecular weight units are assumed to be Daltons.
Mw : Weight average molecular weight of Dextran fractions.
Mn : Number average molecular weight of Dextran fractions.
