Diethoxymethylsilane, DEMS
Sandra Forbes
Product Manager

Recent Literature

Chiral rhodium-bis(oxazolinyl)phenyl complexes catalyze the conjugate hydrosilylation of 3,3-diarylacrylate derivatives to prepare optically active 3,3-diarylpropanoate derivatives in high yields and high enantioselectivities.
K. Itoh, A. Tsuruta, J.-i. Ito, Y. Yamamoto, H. Nishiyama, J. Org. Chem., 2012, 77, 10914-10919.
DOI: 10.1021/jo302357b
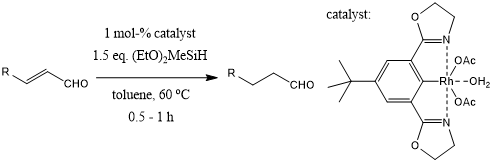
Selective conjugate reductions of α,β-unsaturated aldehydes were achieved in the presence of rhodium(bisoxazolinylphenyl) complexes as catalysts and alkoxyhydrosilanes as reducing agents.
Y. Kanazawa, H. Nishiyama, Synlett, 2006, 3343-3345.
In a Pd(II)-catalyzed enantioselective Markovnikov hydrooxygenation of unactivated terminal alkenes using a substituted pyridinyl oxazoline (Pyox) ligand, a (EtO)2MeSiH/BQ redox system is vital for the highly selective and efficient hydrooxygenation. This method provides efficient access to a broad range of optically pure alcohol esters from easily available alkenes with excellent enantioselectivities.
X. Yang, X. Li, P. Chen, G. Liu, J. Am. Chem. Soc., 2022, 144, 7972-7977.
DOI: 10.1021/jacs.2c02753
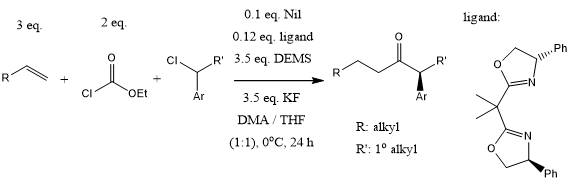
A nickel-catalyzed, multicomponent regio- and enantioselective hydroformylation and carbonylation using chloroformate as a safe CO source provides a wide variety of unsymmetrical dialkyl ketones bearing a functionalized α-stereocenter, including enantioenriched chiral α-aryl ketones and α-amino ketones.
J. Chen, S. Zhu, J. Am. Chem. Soc., 2021, 143, 14089-14096.
DOI: 10.1021/jacs.1c07851
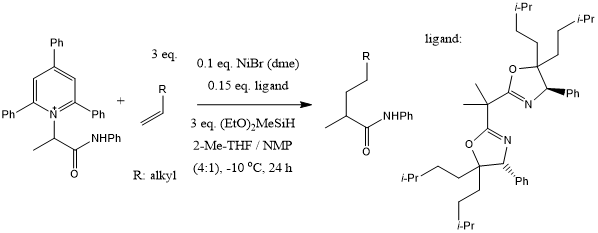
A sterically encumbered bis(oxazoline) ligand backbone enables a Ni-catalyzed enantioselective deaminative alkylation of amino acid and peptide derivatives with unactivated olefins. This protocol is distinguished by its broad scope and generality across a wide number of counterparts, even in the context of late-stage functionalization.
S.-Z. Sun, Y.-M. Cai, D.-L. Zhang, J.-B. Wang, H.-Q. Yao, X.-Y. Rui, R. Martin, M. Shang, J. Am. Chem. Soc., 2022, 144, 1130-1137.
DOI: 10.1021/jacs.1c12350
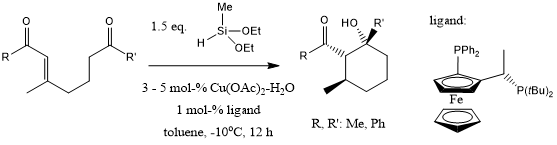
Treatment of β,β-disubstituted-α,β-unsaturated ketones bearing a ketone residue with in situ generated, catalytic CuH ligated by a nonracemic ligand leads to cyclic aldol products with three newly created adjacent chiral centers. Excellent diastereoselectivities and enantioselectivities are obtained for several examples studied.
B. H. Lipshutz, B. Amorelli, J. B. Unger, J. Am. Chem. Soc., 2008, 130, 14378-14379.
DOI: 10.1021/ja8045475
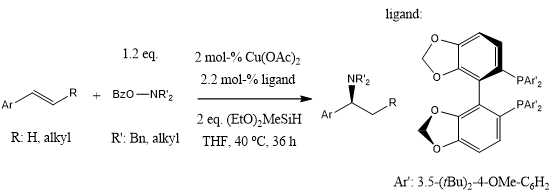
A CuH-catalyzed hydroamination of alkenes using an amine transfer reagent and a silane provides chiral amines with high efficiency and stereoselectivity. However, the current technology has been limited to dialkylamine transfer reagents (R2NOBz). A modified type of monoalkylamine transfer enabled the synthesis of chiral secondary amines, including those derived from amino acid esters, carbohydrates, and steroids.
D. Niu, S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 9716-9721.
DOI: 10.1021/jacs.5b05446
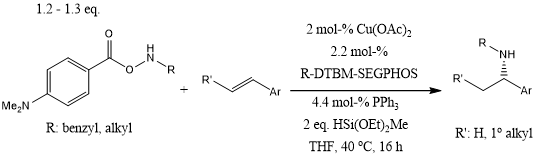
A highly enantio- and regioselective copper-catalyzed hydroamination reaction of alkenes with hydroxylamine esters in the presence of diethoxymethylsilane enables the conversion of a wide variety of substituted styrenes, including trans-, cis-, and β,β-disubstituted styrenes, to yield α-branched amines. In addition, aliphatic alkenes gave exclusively the anti-Markovnikov hydroamination products.
S. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 15746-15749.
DOI: 10.1021/ja4092819
S. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 15746-15749.
DOI: 10.1021/ja4092819
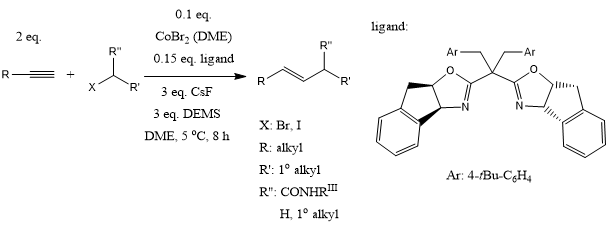
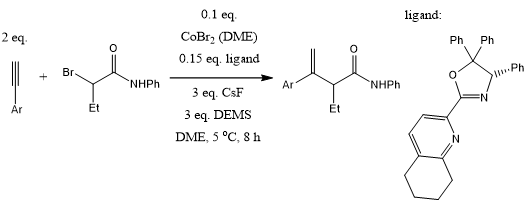
In a ligand-controlled cobalt-catalyzed regiodivergent alkyne hydroalkylation of terminal alkynes with alkyl halides, use of bisoxazoline and pyridine-oxazoline ligands provided (E)-1,2-disubstituted and 1,1-disubstituted alkenes with high E/Z stereoselectivity and regioisomeric ratio. Internal alkynes provide trisubstituted alkenes. The methods show excellent functional group compatibility.
Y. Li, D. Liu, L. Wan, J.-Y. Zhang, X. Lu, Y. Fu, J. Am. Chem. Soc., 2022, 144, 13961-13972.
DOI: 10.1021/jacs.2c06279
In a ligand-controlled cobalt-catalyzed regiodivergent alkyne hydroalkylation of terminal alkynes with alkyl halides, use of bisoxazoline and pyridine-oxazoline ligands provided (E)-1,2-disubstituted and 1,1-disubstituted alkenes with high E/Z stereoselectivity and regioisomeric ratio. Internal alkynes provide trisubstituted alkenes. The methods show excellent functional group compatibility.
Y. Li, D. Liu, L. Wan, J.-Y. Zhang, X. Lu, Y. Fu, J. Am. Chem. Soc., 2022, 144, 13961-13972.
DOI: 10.1021/jacs.2c06279
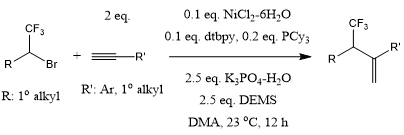
A nickel-catalyzed hydrotrifluoroalkylation of terminal alkynes provides allylic trifluoromethyl terminal alkenes with high efficiency, broad substrate scope, and favorable functional group compatibility. The combination of nitrogen and phosphine ligands, especially electron-rich ones, plays an indispensable role in the course of the reaction.
T. Zhang, Y.-W. Zuo, R.-X. Jin, Y.-F. Zhang, B.-B. Wu, X.-S. Wang, Org. Lett., 2023, 25, 3578-3584.
DOI: 10.1021/acs.orglett.3c01237
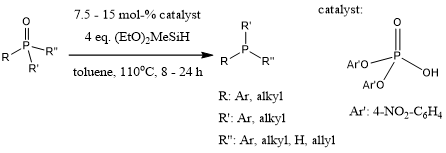
Unprecedented chemoselective reductions of phosphine oxides to phosphines with inexpensive silanes proceed smoothly in the presence of catalytic amounts of specific phosphoric acid esters. The reaction tolerates ketones, aldehydes, olefins, nitriles, and esters under the optimized conditions.
Y. Li, L.-Q. Lu, S. Das, S. Pisiewicz, K. Junge, M. Beller, J. Am. Chem. Soc., 2012, 134, 18325-18329.
DOI: 10.1021/ja3069165
A Ni-H-catalyzed hydroalkylation of vinylsilanes and -germanes as well as allylsilanes with unactivated alkyl iodides proceeds with anti-Markovnikov selectivity to deliver the linear regioisomer. Mechanistic control experiments support a radical mechanism, and a competition experiment reveals that the chemoselectivity is in favor of the vinyl over the allyl group.
D. Brösamlen, M. Oestreich, Org. Lett., 2023, 25, 5319-5323.
DOI: 10.1021/acs.orglett.3c01881
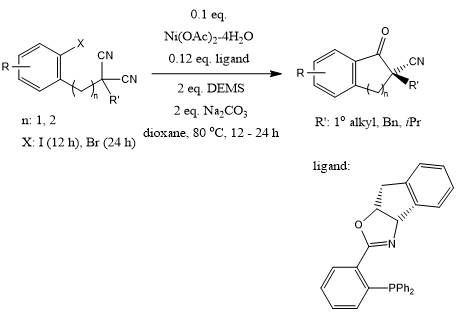
Enantioenriched CN-containing all-carbon quaternary stereocenters can be assembled by desymmetrizing cyclization of aryl/alkenyl halide-tethered malononitriles. The use of a silane reductant is crucial to the enantioselectivity and reactivity.
Z.-H. Chen, R.-Z. Sun, F. Yao, X.-D. Hu, L.-X. Xiang, H. Cong, W.-B. Liu, J. Am. Chem. Soc., 2022, 144, 4776-4782.
DOI: 10.1021/jacs.2c01237
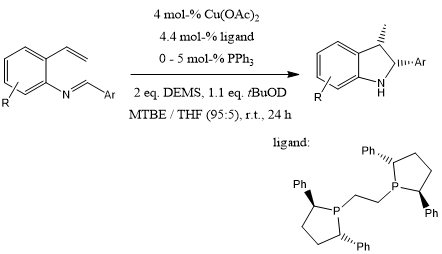
A diastereo- and enantioselective CuH-catalyzed method for the preparation of highly functionalized indolines offers mild reaction conditions and high degree of functional group compatibility. This method is highly valuable for the synthesis various cis-2,3-disubstituted indolines in high yield and enantioselectivity.
E. Ascic, S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 4666-4669.
DOI: 10.1021/jacs.5b02316
Quoted from:
https://www.organic-chemistry.org/chemicals/reductions/diethoxymethylsilane-dems.shtm
Aladdinsci: https://www.aladdinsci.com/