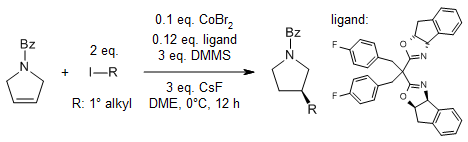
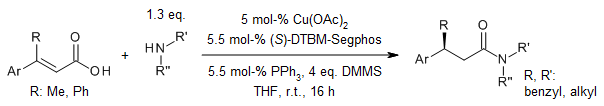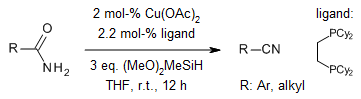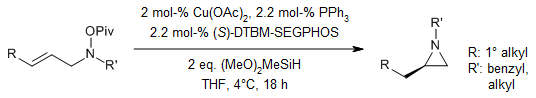Dimethoxymethylsilane, DMMS

Sandra Forbes
Product Manager
Recent Literature

When you use a special type of copper hydride as a catalyst to reduce a certain kind of acid called α,β-unsaturated carboxylic acids, it can produce different kinds of saturated β-chiral aldehydes in good amounts. These aldehydes are very special because they have high levels of something called enantioselectivity and can tolerate a wide range of functional groups. It's like they're more picky and versatile. Scientists also think there's a step in this process where a thing called a ketene intermediate is involved.
Y. Zhou, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 606-609.
DOI: 10.1021/jacs.7b12260
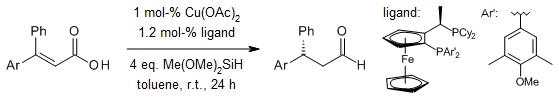
Using a copper hydride catalyst, we can reduce a special type of acid called α,β-unsaturated carboxylic acids to get different kinds of saturated β-chiral aldehydes. These aldehydes are produced in good amounts, and they have high levels of enantioselectivity, meaning they are very specific. Plus, they can tolerate a wide range of functional groups. Scientists also suggest that there's a step in this process where a ketene intermediate is formed.
Y. Zhou, J. S. Bandar, R. Y. Liu, S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 606-609.
DOI: 10.1021/jacs.7b12260
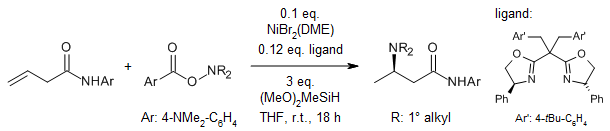
Using a copper hydride (CuH) catalyst, we can directly make β-chiral amides from α,β-unsaturated carboxylic acids and secondary amines under easy reaction conditions. This process can handle many different functional groups in the β-position, including some special types of rings called heteroarenes. After that, we can use an iridium catalyst in the same container to reduce the β-chiral amides into γ-chiral amines.
A. Link, Y. Zhou, S. L. Buchwald, Org. Lett., 2020, 22, 5666-5670.
DOI: 10.1021/acs.orglett.0c02064
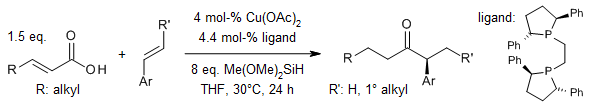
Using a special copper hydride (CuH) catalyst, we can directly connect α,β-unsaturated carboxylic acids with aryl alkenes to make chiral α-aryl dialkyl ketones. This reaction can handle many different kinds of substitutions on the starting materials, sensitive functional groups, and even special rings called heterocycles.
Y. Zhou, J. S. Bandar, S. L. Buchwald, J. Am. Chem. Soc., 2017, 139, 8126-8129.
DOI: 10.1021/jacs.7b04937
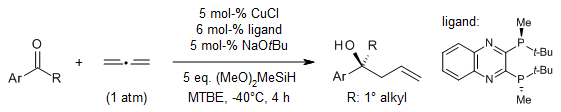
By mixing allene gas with cheap and environmentally friendly hydrosilanes, we can do special reactions called enantioselective ketone allylation without using a lot of allylmetal reagent. This process is made possible by copper salts and easily available ligands. It doesn't need special equipment or high pressure, and it can handle many different kinds of functional groups.
R. Y. Liu, Y. Zhou, Y. Yang, S. L. Buchwald, J. Am. Chem. Soc., 2019, 141, 2251-2256.
DOI: 10.1021/jacs.8b13907
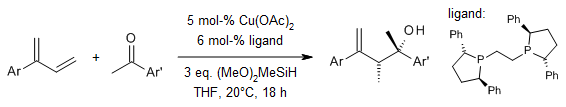
Using a special type of copper called (R,R)-Ph-BPE ligated Cu, we can do a special reaction called allylation of ketones with 1,3-dienes. This reaction needs hydrosilane and happens under easy conditions. It's very good at handling different kinds of functional groups. Instead of using a lot of allylmetal reagents, we use 1,3-dienes which are like hidden allylic nucleophiles. This helps us make chiral homoallylic tertiary alcohols.
B. Fu, X. Yuan, Y. Li, Y. Wang, Q. Zhang, T. Xiong, Q. Zhang, Org. Lett., 2019, 21, 3576-3580.
DOI: 10.1021/acs.orglett.9b00979
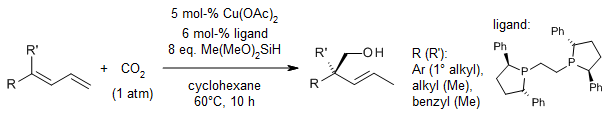
Using a very special copper catalyst, we can take 1,3-dienes and add CO2 to them in a special way to make chiral all-carbon acyclic quaternary stereocenters. These are special types of carbon centers that have four different groups attached to them. The reaction works well with many different kinds of 1,3-dienes, even ones with two different groups attached to the same carbon, and even with a special type called 1,3,5-triene. It's very good at choosing the right way to do the reaction and can handle many different kinds of functional groups.
X.-W. Chen, L. Zhu, Y.-Y. Gui, K. Jing, Y.-X. Jiang, Z.-Y. Bo, Y. Lan, J. Li, D.-G. Yu, J. Am. Chem. Soc., 2019, 141, 18825-18835.
DOI: 10.1021/jacs.9b09721
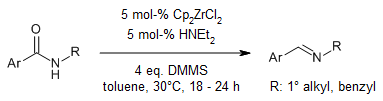
Using zirconocene hydride, we can do a gentle reaction to partly reduce both secondary and tertiary amides into imines. When we use secondary amides, we can get a lot of different imines in very good amounts and with great chemoselectivity. And if we have tertiary amides and add a primary amine, we can also do a reductive transamination at room temperature to get imines.
R. A. Kehner, G. Zhang, L. Bayeh-Romero, J. Am. Chem. Soc., 2023, 145, 4921-4927.
DOI: 10.1021/jacs.2c11786
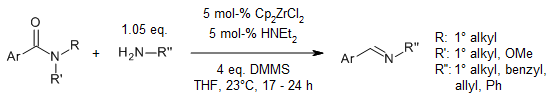
Zirconocene hydride is a special chemical that helps us do a gentle reaction to partly reduce amides into imines. We can use this method for both secondary and tertiary amides. When we use secondary amides, we can get a lot of different imines in good amounts and with great control over which ones we get. And if we have tertiary amides and add a special type of amine called a primary amine, we can also do a special reaction called reductive transamination to get imines, and all this can happen at room temperature.
R. A. Kehner, G. Zhang, L. Bayeh-Romero, J. Am. Chem. Soc., 2023, 145, 4921-4927.
DOI: 10.1021/jacs.2c11786
Using a copper-hydride catalyst to do a special dehydration reaction with primary amides is a cost-effective way to make nitriles. This reaction skips over a hard step called 1,2-siloxane elimination and can happen at room temperature. It can also handle many different kinds of functional groups that are sensitive to metals, acids, or bases.
R. Y. Liu, M. Bae, S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 1627-1631.
DOI: 10.1021/jacs.8b00643
We have a direct way to make alkyl-substituted chiral aziridines from simple starting materials. We use easily available allylic hydroxylamine esters and do a special reaction called intramolecular hydroamination with copper hydride as a catalyst. This reaction gives us the aziridine products in good to excellent amounts and with high control over the shape and arrangement of the molecules. The final products are very rich in one type of chirality.
H. Wang, J. C. Yang, S. L. Buchwald, J. Am. Chem. Soc., 2017, 139, 8428-8431.
DOI: 10.1021/jacs.7b04816
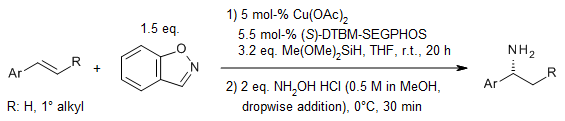
Using 1,2-benzisoxazole as a useful source of electrophilic primary amines, we can do a gentle and general reaction called hydroamination of alkenes and alkynes with copper hydride as a catalyst to make primary amines. This method lets us make a wide variety of chiral α-branched primary amines and linear primary amines.
S. Guo, J. C. Yang, S. L. Buchwald, J. Am. Chem. Soc., 2018, 140, 15976-15984.
DOI: 10.1021/jacs.8b10564
Using a special Ni catalyst, we can do a highly selective reaction called hydroamination on easily available alkenes that have weakly coordinating native amides or esters. This reaction gives us β- or γ-amino acid derivatives and 1,2- or 1,3-diamines, no matter if the alkenes are at the end or in the middle of the molecule. It also works with a wide range of amine coupling partners. This gentle reaction is great for adding functional groups to molecules at the final stages of their synthesis.
C. Lee, H.-J. Kang, H. Seo, S. Hong, J. Am. Chem. Soc., 2022, 144, 9091-9100.
DOI: 10.1021/jacs.2c02343
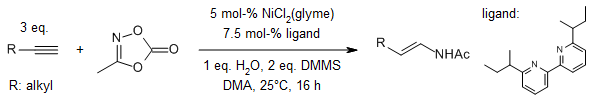
Using a NiH catalyst, we can do a reaction called hydroamidation of alkynes with dioxazolones to easily make secondary enamides. We can choose between (E)-anti-Markovnikov or Markovnikov selectivity for the products. This reaction works well for both terminal and internal alkynes and can handle a range of functional groups. It's important to have H2O present to make the catalyst work efficiently.
X. Lyu, J. Zhang, D. Kim, S. Seo, S. Chang, J. Am. Chem. Soc., 2021, 143, 5867-5877.
DOI: 10.1021/jacs.1c01138
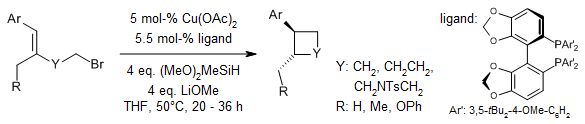
Using a copper hydride catalyst, we can do an enantioselective reaction called intramolecular hydroalkylation on styrenes that have halides attached to them. This allows us to make cyclobutanes, cyclopentanes, indanes, and six-membered N- and O-heterocycles that are enriched with one type of chirality.
Y.-M. Wang, N. C. Bruno, A. L. Placeres, S. Zhu, S. L. Buchwald, J. Am. Chem. Soc., 2015, 137, 10524-10527.
DOI: 10.1021/jacs.5b07061
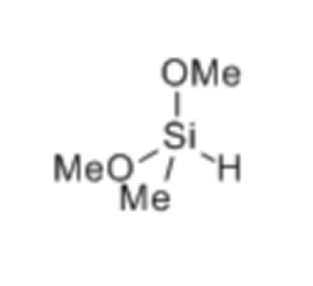
We can use special catalysts to do highly efficient and selective reactions called hydroalkylation on 3-pyrrolines that are easily available. With a Co catalyst, we get chiral C2-alkylated pyrrolidines. And with a Ni catalyst, we get C3-alkylated pyrrolidines. These methods are easy to use because they require catalysts, chiral BOX ligands, and reagents that are readily available.
X. Wang, J. Xue, Z.-Q. Rong, J. Am. Chem. Soc., 2023, 145, 15456-15464.
DOI: 10.1021/jacs.3c03900
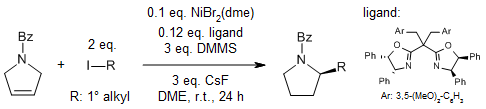
Using efficient catalysts, we can selectively modify 3-pyrrolines that are easily accessible to make chiral C2-alkylated pyrrolidines with a Co catalyst, and C3-alkylated pyrrolidines with a Ni catalyst. The good thing is that these methods require catalysts, chiral BOX ligands, and reagents that are readily available.
X. Wang, J. Xue, Z.-Q. Rong, J. Am. Chem. Soc., 2023, 145, 15456-15464.
DOI: 10.1021/jacs.3c03900
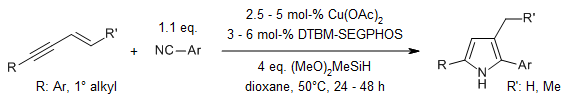
Using an efficient copper hydride (CuH) catalyst, we can do a reaction that combines enynes and nitriles to make polysubstituted N-H pyrroles. These pyrroles have a wide range of functional groups and are produced in good yields with high selectivity. The Cu-based catalyst helps with both the initial coupling step and the subsequent cyclization step.
Y. Zhou, L. Zhou, L. T. Jesikiewicz, P. Liu, S. L. Buchwald, J. Am. Chem. Soc., 2020, 142, 9908-9914.
DOI: 10.1021/jacs.0c03859
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/dimethoxymethylsilane.shtm
For more information, visit our website: www.aladdinsci.com.