Electrochemical Allylic C-H Oxidation with N-Hydroxytetrachlorophthalimide (TCNHPI)

Introduction
Professor Phil Baran and his team have created a new reagent called N-Hydroxytetrachlorophthalimide (H401609), which offers a cost-effective, scalable, and safe alternative for commonly used chemical transformations such as allylic oxidations, as well as Negishi and Suzuki-Miyaura cross-coupling reactions. This reagent eliminates the need for toxic chemicals or precious metals, making it suitable for large-scale and industrial applications.2

Advantages
● A redox-active reagent efficiently accepts electrons during various oxidative transformations, making it highly effective1,3
● Thanks to its electron-withdrawing groups (-Cl), it outperforms N-hydroxyphthalimide as a mediator in synthesizing natural products2
● This reagent can substitute toxic or costly catalysts in widely-used synthetic reactions, paving the way for its industrial-scale application
Representative Applications
1. TCNHPI (H401609) has been utilized in the production of various redox-active esters through its reaction with alkyl acids.1,4 Below are several examples of redox-active esters derived from TCNHPI, synthesized using dichloromethane at room temperature.
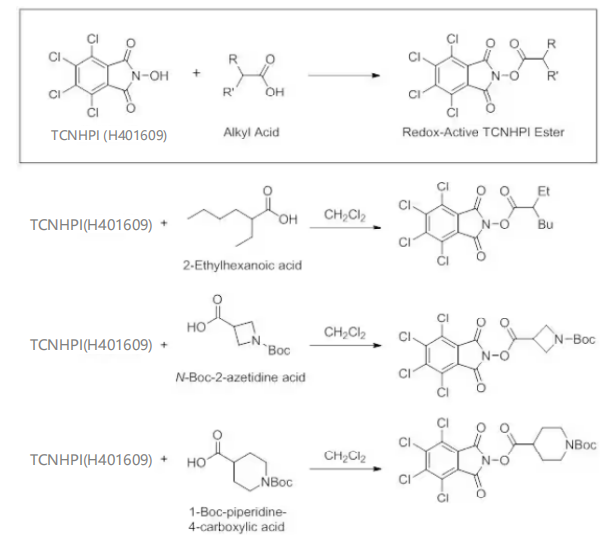
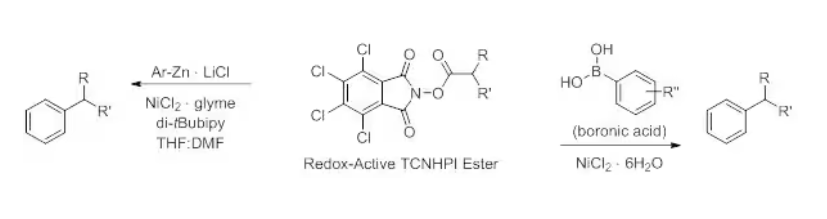
TCNHPI-derived esters readily undergo cross-coupling with aryl zinc reagents1 and are ideal coupling partners for Suzuki coupling reactions.4
2. TCNHPI can serve as a mediator in allylic C-H oxidation reactions, which are crucial for synthesizing natural products. Baran and his team have provided 40 examples to illustrate this, including compounds derived from steroids and triterpenes.2
3. TCNHPI functions as a catalyst in a range of electrochemical allylic oxidation reactions. With its high oxidation potential of 0.87 V versus Ag/AgCl, it efficiently produces a highly energetic and reactive tetrachlorophthalimido N-oxyl radical during the oxidation process.2
The step-by-step mechanism for the electrochemical allylic oxidation of an olefin using TCNHPI is outlined as follows:2
a) TCNHPI reacts with pyridine to undergo deprotonation, resulting in the formation of the tetrachlorophthalimido N-oxyl anion.
b) This anion undergoes anodic oxidation, leading to the generation of the tetrachlorophthalimido N-oxyl radical species.
c) The olefin compound (I) then undergoes hydrogen abstraction by the radical, which regenerates TCNHPI and produces a stable allylic radical (II).
d) The allylic radical (II) reacts with t-BuOO, which is electrochemically generated from t-BuOOH, to form the allylic peroxide (III).
e) Finally, the removal of t-BuOH from (III) results in the formation of the enone (IV).
Conclusions
In summary, H401609 is an economical and scalable stoichiometric reagent that facilitates crucial reactions like allylic oxidation and Ni-catalyzed cross-coupling of carboxylic acids with boronic acids. Given the widespread commercial availability of boronic acids, this technology and reagent will necessitate a reevaluation of the design and synthesis of molecules, regardless of their size and complexity.
Reference
1. Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan C, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. 2016. Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters. J. Am. Chem. Soc.. 138(7):2174-2177. https://doi.org/10.1021/jacs.6b00250
2. Horn EJ, Rosen BR, Chen Y, Tang J, Chen K, Eastgate MD, Baran PS. 2016. Scalable and sustainable electrochemical allylic C-H oxidation. Nature. 533(7601):77-81. https://doi.org/10.1038/nature17431
3. Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. 2016. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 352(6287):801-805. https://doi.org/10.1126/science.aaf6123
4. Wang J, Qin T, Chen T, Wimmer L, Edwards JT, Cornella J, Vokits B, Shaw SA, Baran PS. 2016. Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids. Angew. Chem. Int. Ed.. 55(33):9676-9679. https://doi.org/10.1002/anie.201605463
Aladdin:https://www.aladdinsci.com



