Flow cytometry analysis

When a suspension of cells is passed through a flow cytometer, the sheath fluid focuses the cells hydro-dynamically, causing them to pass in a single file through a small nozzle. The resulting tiny stream of fluid carries the cells one by one past a laser light, as shown in Figure 1.

Figure 1. Flow cytometry diagram giving an overview of the flow cytometer. A sheath fluid focuses the cell suspension, causing the cells to pass through a laser beam one at a time. Forward and side scattered light is detected, as well as fluorescence emitted by stained cells.
The multicolor flow cytometer
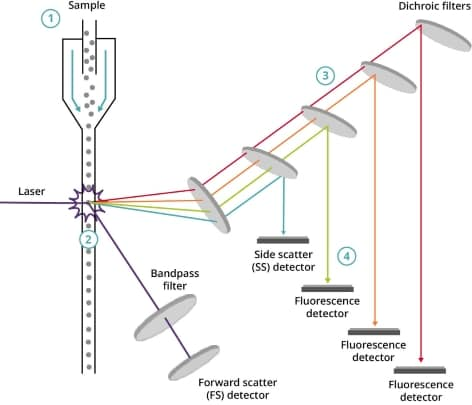
Figure 2. Overview of basic multicolour flow cytometry technology
When a sample is introduced into the flow chamber of a multicolour flow cytometer, it enters the fluidics system and is separated into individual cells using a process called hydrodynamic focusing. This system uses controlled fluid flow to narrow the diameter and align the sample into a single file, thus separating the cells.
As the laser scans each cell, the instrument logs it as an event. For each event, both forward scatter and side scatter are recorded. If a cell has fluorescence labeling, the laser stimulates the fluorophore, and the emitted light is measured as fluorescence intensity.
To detect the specific wavelength emitted by a fluorophore, the emitted light passes through a series of mirrors and filters until it reaches the designated photomultiplier tube (PMT) detector. PMTs detect fluorescence at a specific wavelength.
Optical filters prevent some wavelengths from passing through and permit others. A dichroic filter works as a mirror at an angle, transmitting certain wavelengths while reflecting others. The classification and arrangement of dichroic filters enable the detection of numerous signals at the same time.
Measurement of forward and side scattered light
All particles or cells passing through the beam scatter laser light. This is measured as forward scatter by detectors located in front of the light beam and as side scatter by detectors located to the side of the light beam (refer to Figure 3).
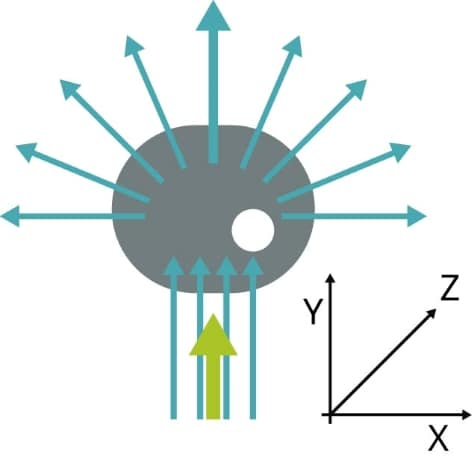
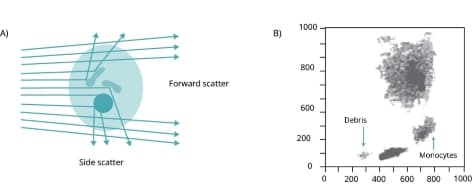
Figure 3. When the green laser interacts with the cell, light scatters. The direction in which the cell scatters light shows its size and granularity.
FS indicates the cell size, and SS is proportional to the granularity of the cells. This means that variations in size and granularity enable identification of different cell populations (Figure 4).
Figure 4. a) Flow cytometry measures laser light's forward scatter (FS) and side scatter (SS) to reflect cell size and granularity. b) A typical plot illustrates the distinguishable immune cell types based on FS and SS data.
An example illustrating this principle is analyzing blood samples on the flow cytometer.
-Large and highly granular granulocyte cells are detected as a significant population with high side scatter (SS) and forward scatter (FS).
-Monocytes, being large cells with less granularity, are identified as a separate population with a higher FS but lower SS.
-Smaller lymphocytes and lymphoblasts form a distinct population with lower FS and SS since they are not granular cells.
Therefore, it is possible to classify these cells into distinct groups solely based on their FS and SS values (refer to Figure 5).
Figure 5. Flow cytometry diagram showing FS vs. SS. Each dot on the graph represents a single cell that has been analyzed by the flow cytometer. Differences in the size and granularity of the different cell populations determine their unique position.
Measurement of scattered light and fluorescence
As well as measuring forward and side scattered light from all cells or particles in a sample, fluorescence detectors within the flow cytometer measure the fluorescence emitted from positively stained cells or particles. For example, these could be stained with a fluorescently labeled antibody against a particular protein or a fluorescent ligand that binds a specific structure such as DNA. These fluorophores (or fluorochromes) emit light when excited by a laser with the corresponding excitation wavelength.
FS and SS light as well as fluorescence from stained cells are split into specific wavelengths and directed through a series of filters and mirrors within the flow cytometer towards photomultiplier tubes (PMTs) which are used as sensors. The electrical signal (voltage) is generated by the PMTs when a photon's energy is converted into it. To ensure that each sensor detects fluorescence at a particular wavelength, the fluorescent light is filtered.
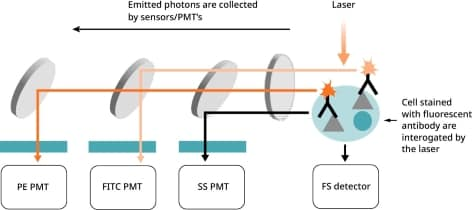
In Figure 6, the FITC channel PMT detects light emitted from FITC at around 519 nm wavelength. The PE channel PMT detects light emitted from PE at 575 nm wavelength. Additionally, each PMT detects any other substances present in the sample emitting light at a similar wavelength to the fluorophore that it is detecting.
Figure 6. Fluorescent antibody-stained cells pass by the laser.
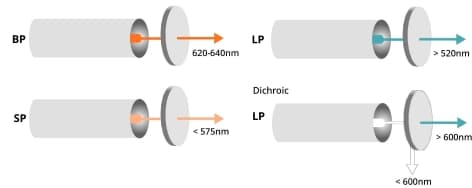
The flow cytometer employs various filters to direct photons of the correct wavelength to each PMT (as seen in Figure 7). Short-pass (SP) filters transmit photons below a specified wavelength, while long-pass (LP) filters transmit photons above a specified wavelength.
Dichroic filters/mirrors, specifically dichroic LP mirrors, are positioned at a 45° angle to the light beam and redirect light of undesired wavelengths rather than completely blocking it. For instance, when the wavelength of photons exceeds a certain value, they travel straight through a long-pass dichroic filter. Conversely, when the wavelength of photons is lower than the specified value, they reflect at a 90° angle.
Figure 7. Band pass (BP), short pass (SP), and dichroic filters in the flow cytometer.
Reference
1.McKinnon KM. Flow Cytometry: An Overview. Curr Protoc Immunol 2018;120:5.1.1-5.1.11
2.Holmberg-Thyden S, Gronbaek K, Gang AO, et al. A user’s guide to multicolor flow cytometry panels for comprehensive immune profiling. Analytical Biochemistry 2021;627:114210
3.Maciorowski Z, Chattopadhyay PK, Jain P. Basic Multicolor Flow Cytometry. Curr Protoc Immunol 2017;117:5.4.1-5.4.38