Flow Cytometry

Flow cytometry enables the separation of a suspended sample of cells or particles through a thin, rapidly moving liquid stream. As the sample passes through a laser, it can detect the size, granularity, and fluorescent properties of each individual cell or particle. This technique allows for the identification of heterogeneous biological samples, for example, T-cells in blood or photosynthetic microbes in seawater. Fluorescence labelled antibodies can be used to target specific surface proteins, providing a more detailed analysis of the sample. For example, activated and non-activated T-cells can be differentiated. In this text, the term "the particle" will refer to any particle or cell.
When a particle passes through an aperture accompanied by an electric current, the alteration in impedance is directly proportional to the size of the particle, and this phenomenon is referred to as the Coulter principle. This becomes particularly useful when the particles are of low conductivity, as in the case of cells. Mack Fulwyler integrated the use of the Coulter principle for counting and sizing particles when he invented the first cell sorter in 1965. Leonard Herzenberg expanded the technique and coined the term "FACS" for fluorescent-activated cell sorting. The acronym FACS is trademarked and owned by Becton Dickinson. Although the term is commonly used by many scientists for sorting and non-sorting applications, it is not a generic term for flow cytometry.
Technology
A sample containing fluorescent labeled particles is connected to a flow cytometer, where hydrodynamic focusing orients the particles into a thin stream surrounded by higher speed fluid (sheet fluid). A laser beam illuminates the stream and a set of detectors are aimed at the same point. The detectors detect light in line with the light beam, known as Forward Scatter (FSC), and perpendicular to it, known as Side Scatter (SSC), as well as one or more sets of fluorescent molecules. FSC corresponds with the particle volume, and SSC depends on the particle's internal complexity. Usually, particles in the range of 0.2 to 150 micrometer that pass through the beam scatter light at a detectable level based on SSC or FSC. The detectors identify scattered light together with fluorescent light emitted from chemicals found in or attached to the particle surface. Brightness fluctuations at each detector (one for each fluorescent emission peak) enable the derivation of various types of information about the physical and chemical structure of each individual particle. Modern flow cytometers often feature multiple lasers and fluorescence detectors, which enable complex experimentation. Acquiring data from samples using a flow cytometer is known as "acquisition," a process handled by a computer connected to the cytometer and software responsible for managing the digital interface. The software can adjust parameters, including PMT voltage for signal amplification and compensation for fluorescent channel leakage, for the test sample. It also displays initial sample information during data acquisition to ensure correct parameter settings. A flow cytometer typically consists of five main components before sample collection, as shown in Figure 1.
A. A flow cell is utilized to align the liquid stream (sheath fluid), carrying the cells/particles, enabling their passage in a single file through the light beam for sensing.
B. A commonly used measuring system today consists of diode lasers with various wavelengths (blue, green, red, and violet) which produce light signals.
C. A detector, typically a Photo Multiplier Tube (PMT), and an Analogue-to-Digital Conversion (ADC) system, produce Forward Scatter (FSC), Side Scatter (SSC), and fluorescence signals that are converted from light into electrical signals. These signals can then be analyzed by a computer.
D. An amplification system (linear or logarithmic)
E. A computer for analysis of the signals
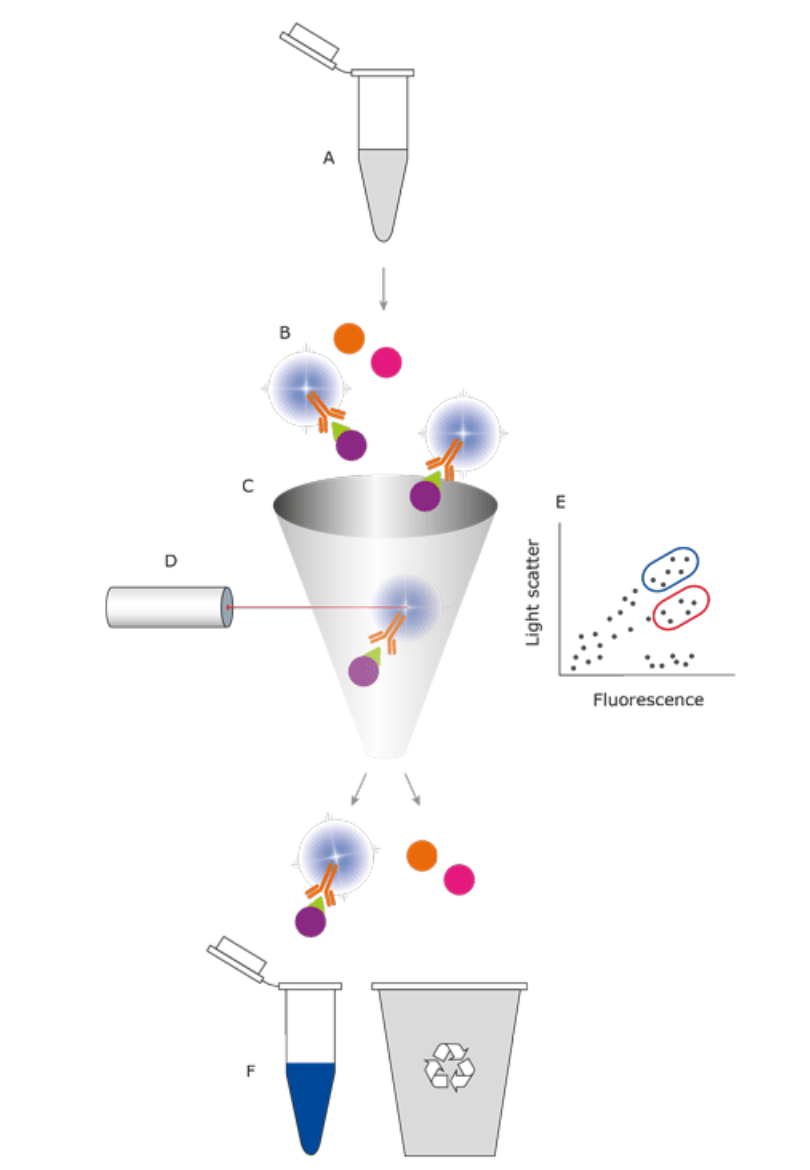
Figure 1 shows the general principle of flow cytometry. A diverse cell sample (A) is labelled with fluorescent antibodies (B) and the particles in the sample are focused in a fluid flow (C). This flow allows only one particle at a time to reach a light source (D). Depending on the particle's nature, light scattering or fluorescence will vary. The resulting signal is amplified and monitored in a computer diagram, where gates around particles of interest can be drawn (blue and red) (E). Certain flow cytometers possess sorting capability, enabling targeted particles with desired traits to be directed into collection containers (F).
Antibodies in Flow Cytometry
Antibodies are essential tools for the accurate analysis of complex biological samples in flow cytometry, as they enable the identification of specific marker proteins on a cell's surface. The more markers employed, the greater the amount of information obtained per cell. The more antibodies employed, the greater the amount of information obtained. Nevertheless, there are limitations to the number of fluorochromes available and the number of lasers and detectors that can be used in a FACS machine. At present, the most advanced flow cytometers carry seven lasers and 49 detectors.
A recent technology called mass cytometry circumvents some of the limitations of fluorochromes; instead, antibodies are labelled with rare earth metals, allowing 40 different antibodies to be detected simultaneously.
Sorting by Flow Cytometry
Fluorescence-activated cell sorting (FACS) is a unique form of flow cytometry that enables the sorting of individual cells or particles based on their detected light scattering. Citations should be consistent and marked clearly. This sorting can be performed either directly into microtitre plates or tubes, one cell at a time. The narrow stream of liquid (known as 'sheet fluid') that carries the cells permits separation, ensuring only one cell reaches the detector at any given time. To enable the isolation of specific cells within the flow, a vibrating mechanism causes the stream of cells to break up into individual droplets. Prior to droplet formation, the flowing liquid is scanned by lasers and detectors that assess its scatter and fluorescence parameters. At the precise point where the stream breaks off into droplets, an electrical field is applied. Based on the immediately preceding fluorescence intensity measurement, a charge is applied and the opposite charge is trapped on the droplet as it breaks out of the stream. The charged droplets subsequently pass through an electrostatic diversion system, which redirects them into tubes or wells located on a microtiter plate.
Specific Examples
Flow cytometry is an indispensable tool in both research and diagnostics. In healthcare, it serves to diagnose various diseases, including blood cancers, while also aiding in clinical decision-making in fields such as transplantation, hematology, tumour immunology and chemotherapy, genetics, and sex pre-selection through sperm sorting. The auto-fluorescent properties of photosynthetic plankton are utilised via flow cytometry in marine biology for quantifying abundance and determining community structure. In protein engineering, the combination of yeast and bacterial display with flow cytometry is used to detect cell surface-bound protein variants with desired properties.
Epitope mapping can be achieved by displaying and sorting bacteria. Antibodies are incubated with a bacteria library, in which each bacterium presents a specific type of peptide on its surface for mapping purposes. Two fluorescent signals are detected: blue fluorophores indicating antibody-peptide binding, and red fluorophores indicating surface expression of the normalization tag binding to the pink control protein. Certain members of the library (Bacteria B and Bacteria C) do not express peptides identified by the antibody, thereby enabling detection solely of the red fluorescence. To isolate the binder from the non-binding peptides background, a gate is drawn around the population of binding and expressing bacteria (highlighted with blue rectangle) and the flow sorter is adjusted to collect these cells into a container (blue tube). Applying the same principle, non-binding peptides can be sorted and collected in a separate container (red tube).
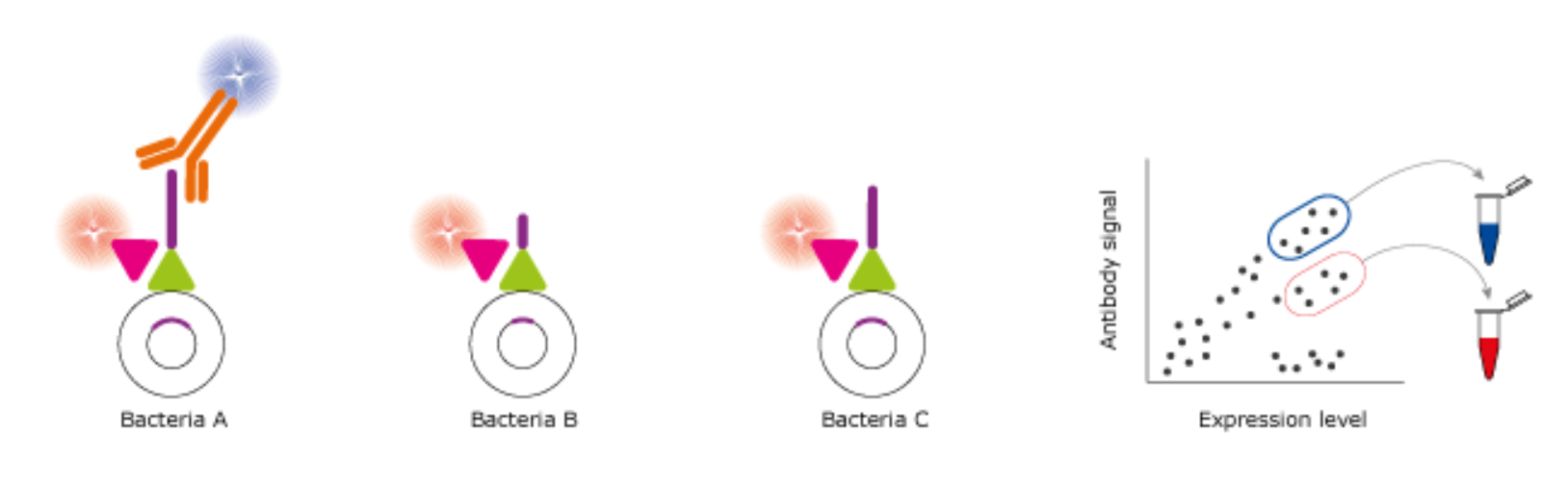
Figure 2. Identification and segregation of cell populations for epitope mapping. Peptides (purple) are showcased on the surface of bacteria and then subjected to incubation with labelled antibodies. Following this, the bacteria are sorted based on their characteristics.
A standard outcome of an epitope mapping setup is displayed in Figure 3. Here, a Human Protein Atlas (HPA) antibody aimed at the cancer protein HER2 is incubated with a peptide library. The bacteria that express peptides of interest are isolated by drawing a gate (1) around them and running the flow sorter in sort-mode to collect them. The gathered sample is analyzed in the flow sorter. Successful sorting is indicated by the enrichment of dots (bacteria) in the same region (2). The bacteria gathered from gate 2 can then be sorted and subjected to DNA sequencing to obtain the epitope peptide sequence expressed on their surface.
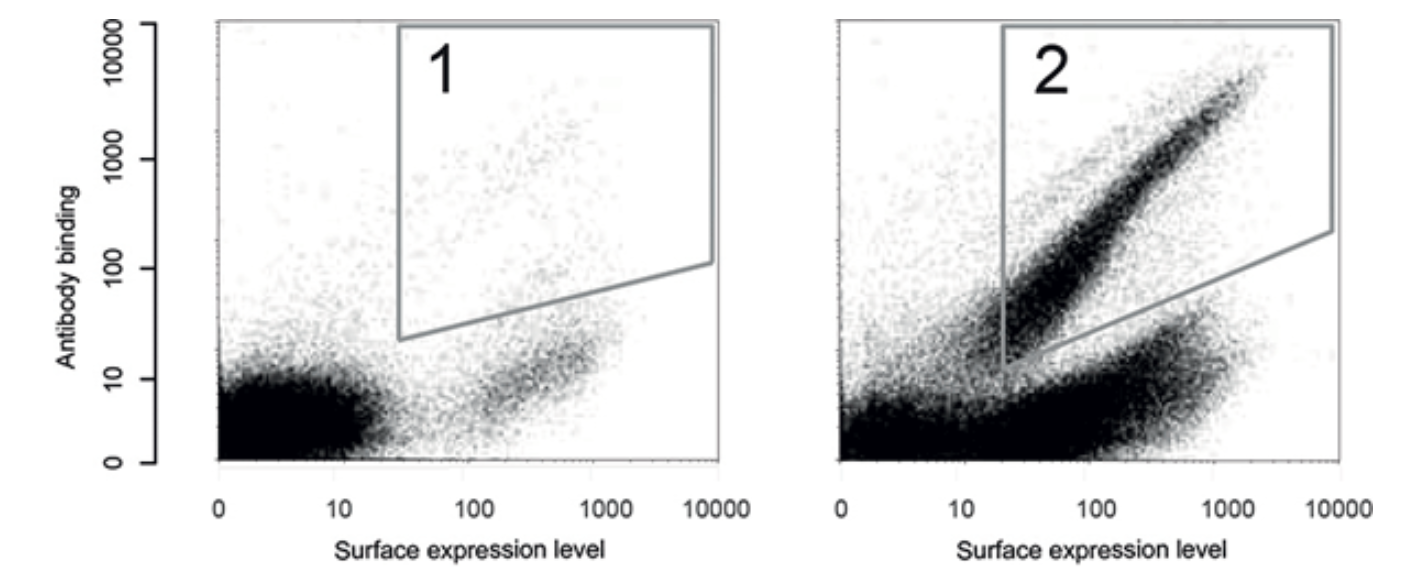
Figure 3. Mapping the epitope of a HER2 antibody using bacterial display and cell sorting. An antibody produced by HPA, which targets the cancer-related protein HER2, is incubated with a library of peptides, and a gating system is used to collect bacteria exhibiting specific properties.
Reference
1.Ornatsky O et al., Highly multiparametric analysis by mass cytometry. J Immunol Methods. (2010)
PubMed: 20655312 DOI: 10.1016/j.jim.2010.07.002
2.Rockberg J et al., Epitope mapping of antibodies using bacterial surface display. Nat Methods. (2008)
PubMed: 19029907 DOI: 10.1038/nmeth.1272
3.Shapiro H, Practical Flow Cytometry, Wiley & Sons Inc 2003: http://www.beckmancoulter.com/wsrportal/wsr/research-and-discovery/products-and-services/flow-cytometry/practical-flow-cytometry/index.htm
4.Flow cytometry, a laser-based, biophysical technology used to count, measure size, and detect properties of particles in suspension: http://en.wikipedia.org/wiki/Flow_cytometry
5.Coulter counter, an apparatus for counting and sizing particles suspended in electrolytes: http://en.wikipedia.org/wiki/Coulter_counter
6.Mass cytometry, a mass spectrometry technique used for the determination of the properties of cells (cytometry): http://en.wikipedia.org/wiki/Mass_cytometry
7.Hydrodynamic focusing uses a liquid to create a tunnel from fluid in which the sample can be injected: http://en.wikipedia.org/wiki/Hydrodynamic_focusing
8.Antibodypedia - An open-access database of publicly available antibodies and their usefulness in various applications: http://www.antibodypedia.com
