Fluorescent probes – brightening the horizon of biomedical research
Product Manager:Harrison Michael

In 1998, the field of medical science marked a major milestone when Robert F. Furchgott, Louis J. Ignarro, and Ferid Murad were awarded the Nobel Prize in Physiology or Medicine for uncovering the role of nitric oxide (NO) as a key signaling molecule in the cardiovascular system. This breakthrough discovery not only highlights the central role of NO in cardiovascular physiology, but also highlights its important role in multiple biological processes such as neural signaling, endothelial function, and immune regulation. NO plays a key role in physiological activities such as vasodilation, neurotransmission, cytotoxic effects, immune response and inflammation.
Nitric oxide synthase (NOS) is a catalytic enzyme that converts arginine to citrulline and NO in the presence of molecular oxygen, tetrahydrobiopterin, NADPH, and xanthine cofactors. Although the active period of NO is short, it can rapidly penetrate the cell membrane and diffuse between cells, which has a significant impact on neuronal transmission, synaptic plasticity, and the regulation of vascular tone. However, the benefits of NO may be offset by its potential toxicity, especially when it forms peroxynitrite, an oxidative free radical that can damage cellular components.
Given the low concentration and transient existence of NO in organisms, real-time monitoring of NO requires a method that is both highly sensitive and specific. Fluorescence microscopy, aided by a suitable NO probe, has become an effective tool to observe the dynamic changes of NO. Recent advances in NO probe design are reflected in the use of o-aromatic diamines to selectively capture and detect NO. For example, 5, 6-diaminofluorescein diacetate (DAF-2-DA) is a novel NO probe, which can be hydrolyzed by cytoplasmic esterase to produce fluorescent substance (DAF-2T) in the presence of NO, thus enabling accurate detection of intracellular NO levels.
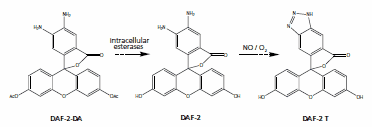
Figure 1:DAF-2-DA probe1
Although previous NO probes, such as 2, 3-diamino-naphthalene (DAN), have shown high sensitivity, their limitations have prompted scientists to develop enhanced probes such as DAN-1 to significantly reduce the leakage problem. Furthermore, the fluorescein and rhodamine based probes overcome the drawbacks of the naphthol based NO probes and provide better biocompatibility, solubility, and spectral properties.
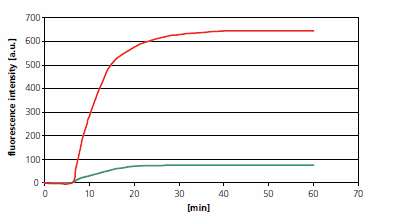
Figure 2:Conversion from DAN-1 to the fluorescent form DAN-1-T was observed after addition of different concentrations of nitric oxide (NO) donor (NONOate). Specifically, when NONOate at a final concentration of 5 mM (represented by green line) or 50 mM (represented by red line) was added, the time-series changes in DAN-1 formation of DAN-1-T were recorded2.
In addition to the detection of NO, the detection techniques of oxygen and reactive oxygen species (ROS) are also crucial for understanding cell signaling pathways and pathological processes. Oxygen probes, as sensitive indicators, can change the fluorescence intensity or emission spectrum after binding to molecular oxygen, and play a key role in a variety of applications such as tissue oxygen monitoring and hypoxia related research. Similarly, ROS probes are important for the study of those diseases characterized by elevated ROS levels, such as inflammation and cancer, necessitating the development of photostable sensors with low autooxidation rates and minimal by-product production.
In summary, the development of advanced detection techniques has greatly advanced our understanding of NO kinetics and REDOX biology, and provided us with insights into physiological processes and pathological conditions. These techniques not only enable real-time visualization of molecular interactions but also open new hopes for targeted therapeutic interventions against NO-related diseases and ROS-related pathologies.
Reference
1. Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, and Nagano T. “Detection and Imaging of Nitric Oxide with Novel Fluorescent Indicators: Diaminofluoresceins,” J. Anal.Chem. 70(13), 2446-2453(1998). https://doi.org/10.1021/ac9801723
2. Kojima H, Sakurai K, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Akaike T, Maeda H, and Nangano T. “Development of a Fluorescent Indicator for the Bioimaging of Nitric Oxide,” J. Biol. Pharm. Bull.. 20(12), 1229-1232(1997). https://doi.org/10.1248/bpb.20.1229
3. Palmer RMJ, Ferrige AG, and Moncada S. “Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor,” J. Nature. 327(6122), 524-526(1987). https://doi.org/10.1038/327524a0
Aladdin:https://www.aladdinsci.com/
