GDC-1971: SHP2 Inhibitor Ushering in a New Era of Cancer Treatment
Sandra Forbes
Product Manager

Introduction

In recent years, protein tyrosine phosphatase SHP2 has become a highly regarded research target in the field of oncology in mediating the RAS-driven MAPK signaling pathway. Its treatment methods are not limited to single drug use, but also can work together with KRAS inhibitors, showing good therapeutic potential. The researchers have initially found that certain similar drugs can bind to the heterogeneous sites, thus keeping the enzyme in a closed and inactive state. It is particularly noteworthy that GDC-1971, formerly known as RLY-1971, as a SHP2 inhibitor, is currently being used in clinical trials in combination with KRAS G12C inhibitor divarasib GDC-6036 to fight against solid tumors driven by KRAS G12C mutations, bringing new hope to the field of tumor treatment.

Synthesis of GDC-1971
During the optimization process of medicinal chemistry, compounds such as GDC-1971 primarily follow two synthetic pathways. Both of these pathways employ similar chemical techniques to adjust the core structure, basic amine, and tetrahydroquinoline regions, effectively combining diverse structural units and thus achieving the optimization and improvement of the compounds.

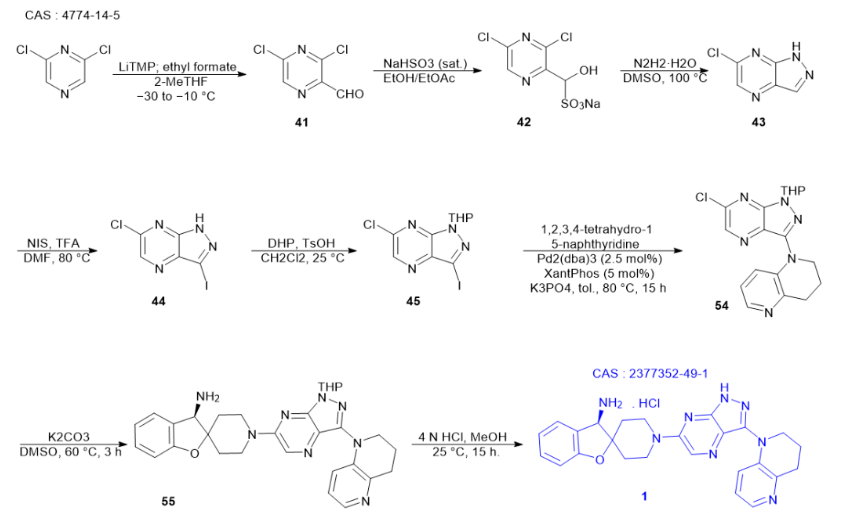
General Synthesis of Analogues
Starting from 2,6-dichloropyrazine, 3,5-dichloropyrazine-2-carbaldehyde can be synthesized through petrochemical treatment and quenching reaction with ethyl formate. Given the instability of this aldehyde, it is preferable to convert it into the corresponding sodium bisulfite adduct form for more effective separation. Furthermore, when this adduct is treated with hydrazine, a mixture of adducts is generated. However, during this process, the corresponding hydrazone undergoes intramolecular cyclization, converting into 6-chloro-1H-pyrazolo[3,4-b]pyrazine. Subsequently, compound 44 is obtained through an iodination process. To obtain the desired chloroiodo intermediate 45, dihydropyran is then used for protection treatment.
In an alkaline environment, the key intermediate 1-(6-chloro-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyrazin-3-yl)-1,2,3,4-tetrahydro-1 undergoes a series of reactions. Firstly, it undergoes palladium-catalyzed coupling with tetrahydroquinoline and Xantphos, resulting in the formation of 5-naphthyridine. Subsequently, this heteroaryl chloride undergoes a nucleophilic aromatic substitution reaction with the bis-hydrochloride salt of (R)-3H-spiro[benzofuran-2,4′-piperidine]-3-amine and diisopropylethylamine in dimethylformamide, ultimately yielding the tetrahydropyran-protected analogue of GDC-1971. For further processing, this salt can be liberated with an aqueous solution of sodium hydroxide and subsequently extracted with 2-methyltetrahydrofuran. This series of steps is intricate and crucial, providing an important intermediate for the preparation of GDC-1971.
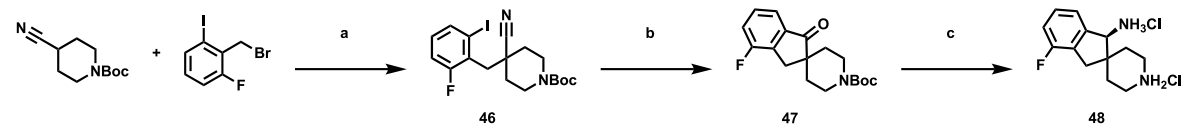
Representative Synthesis of Aryl Spirocyclic Amine Building Blocks
The following scheme provides a detailed description of the synthetic process used to prepare aryl spirocyclic amine structural units for analogues. In this process, asymmetric reduction is first carried out using a chiral ligand such as an Erman reagent, followed by a global deprotection step to obtain the diamine salt. This product can be directly used in nucleophilic aromatic substitution reactions with compounds like IntA, providing a crucial precursor for subsequent synthetic steps.
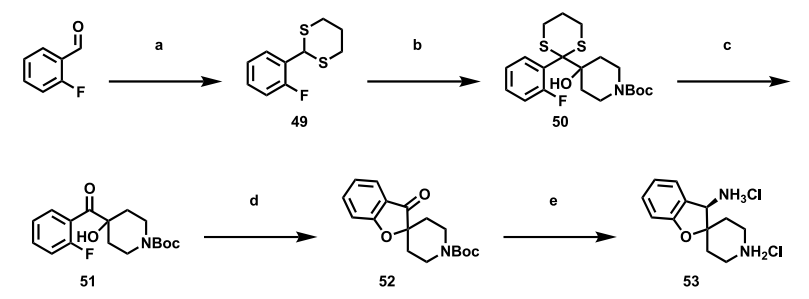
Synthesis of the Spirobenzofuran-Piperidine Ring in GDC-1971
(R)-3H-spiro[benzofuran-2,4′-piperidine]-3-amine, as the crucial basic amine component of GDC-1971, begins its synthesis with 2-fluorobenzaldehyde. Subsequently, through a series of transformation steps, it is successfully converted into the corresponding 1,3-dithiane, laying a solid foundation for subsequent synthetic reactions.
Pharmacological study of GDC-1971
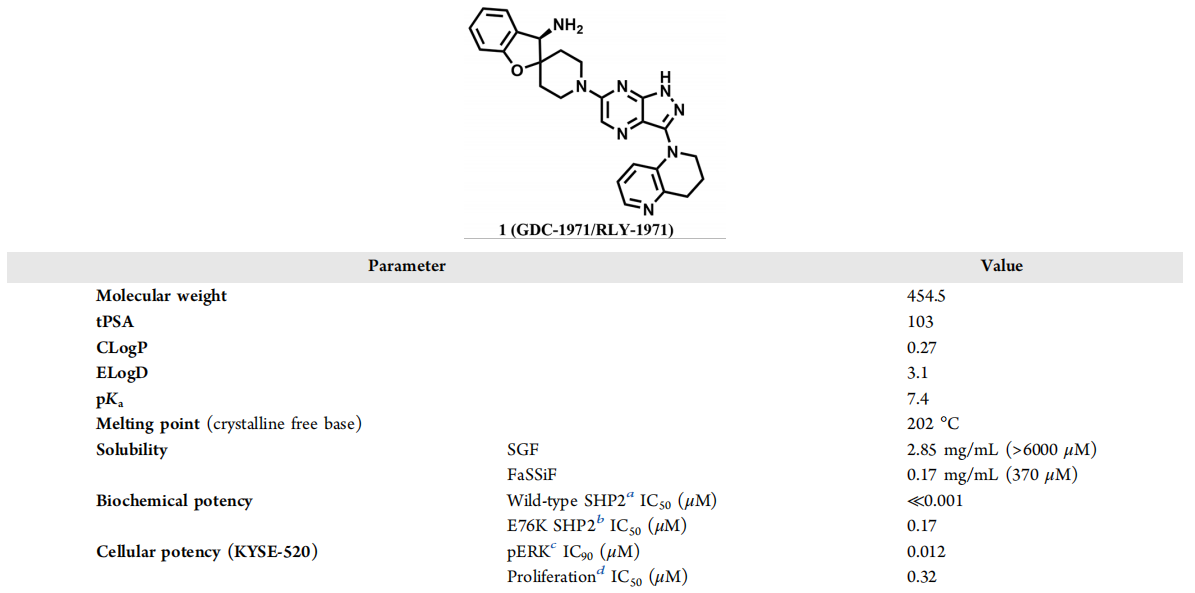
GDC-1971, also known as (R)-1′-(3-(3,4-dihydro-1,5-naphthyridin-1(2H)-yl)-1H-pyrazolo[3,4-b]pyrazin-6-yl)-3H-spiro[benzofuran-2,4′-piperidine]-3-amine, has demonstrated significant inhibitory effects on SHP2 in both biochemical and cellular environments.
Selective parameters of GDC-1971: structure, physicochemical properties, and potency
To more accurately predict the pharmacokinetic (PK) characteristics and effective dose of GDC-1971 in humans, PK assessments were conducted in preclinical species, and in vitro ADME data were collected in preclinical species and human systems to provide comprehensive information on drug metabolism and kinetics.
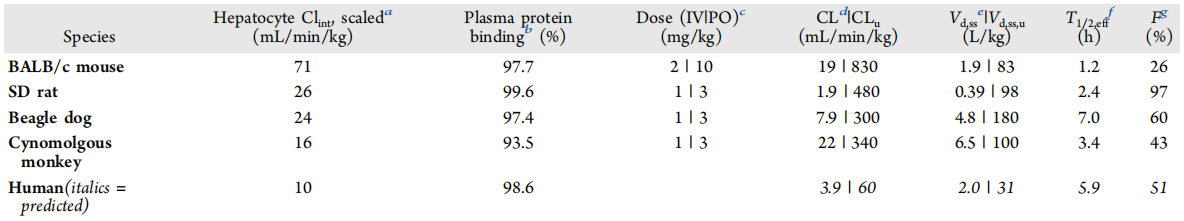
The pharmacokinetic characteristics of GDC-1971 across multiple species
Both in vitro and in vivo efficacy, pharmacokinetic (PK), and non-target activity data of GDC-1971 have demonstrated that the drug can effectively inhibit SHP2 in humans within an appropriate dose range with an acceptable safety margin. Currently, this compound has successfully progressed to further safety and IND-enabling studies and is expected to eventually enter clinical trials. Future research progress and clinical trial results will be disclosed at appropriate times.
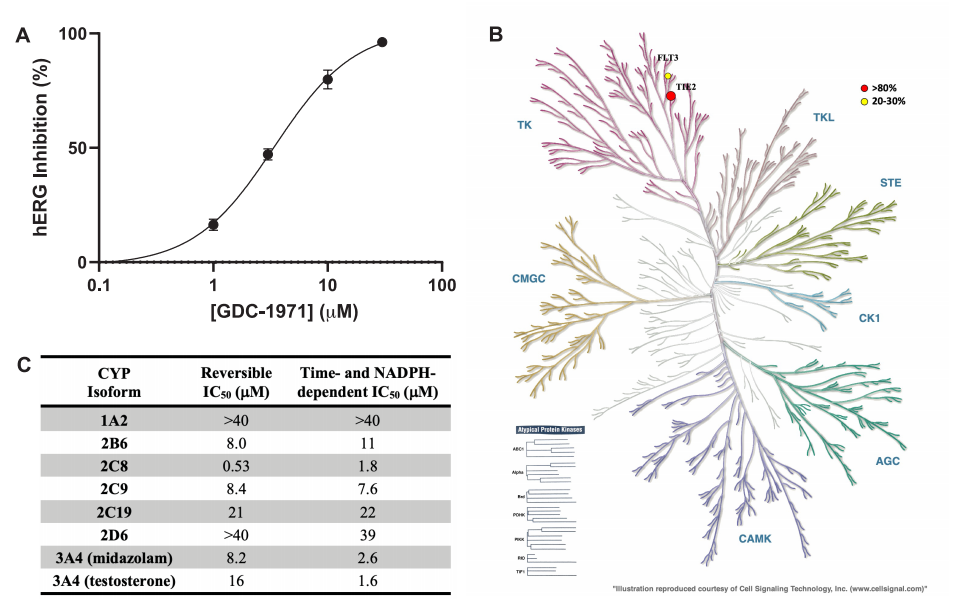
GDC-1971 Off-target Profile: (A) Dose-response curve for functional hERG activity. (B) Dendrogram showing inhibition of a set of kinases. (C) CYP inhibition profile.
Conclusion
In summary, the characteristics exhibited by GDC-1971 during preclinical stages both in vitro and in vivo suggest its significant potential as a clinical trial candidate for testing SHP2 inhibition. Based on the synthetic route detailed in this article, GDC-1971 can be produced in large-scale synthesis using bulk-prepared building blocks, providing a solid foundation for subsequent clinical studies.
References
1. Alexander M. Taylor. et al. Identification of GDC-1971 (RLY-1971), a SHP2 Inhibitor Designed for the Treatment of Solid Tumors. J. Med. Chem. 2023, 66, 19, 13384-13399. https://doi.org/10.1021/acs.jmedchem.3c00483
Aladdin:https://www.aladdinsci.com