Aladdin@Guidelines for the formulation of commonly used buffers for biological experiments

Product Manager:
Harrison Michael
In order to facilitate the experiments of scientific researchers, we have sorted out the buffer formulations commonly used in laboratory basic research in molecular biology, protein biology, microbiology and other aspects for researchers to facilitate access and use.
1. DNA electrophoresis buffer:
(1) Agarose electrophoresis TAE buffer:
①50×TAE storage solution: Tris-base 242 g/L, EDTA·Na2·2H2O 37.2 g/L, glacial acetic acid 57.1 mL, ddH2O added to a measuring cylinder for constant volume, stored at room temperature for use, diluted to 1×when used.
②1×TAE working solution: Tris-base 242g and EDTA·Na2·2H2O 37.2g were weighed in a 1L beaker using an analytical balance. About 800 mL of ddH2O was added to the beaker and dissolved with thorough stirring. 57.1 mL of glacial acetic acid was weighed using a measuring cylinder, the pH was adjusted to 8.3 with NaOH, and the solution was constant volume to 1L with the addition of ddH2O and stored at room temperature.
(2) Tris-boric acid (TBE) buffer:
①5×TBE storage solution: 54g Tris-base, 27.5g boric acid, and 20 mL 0.5mol/L EDTA were weighed, then ddH2O was added to dissolve and mix, the pH was adjusted to 8.0 with NaOH, and the solution volume was set at 1L.
②1×TBE working solution: 5×was used for dilution.
(3) Tris-phosphate (TPE) buffer:
①10×TPE storage solution: 108g Tris-base was weighed, 15.5mL of 85% phosphoric acid and 40 mL of 0.5 mol/L EDTA were weighed using a measuring cylinder, ddH2O was added to dissolve and mix, the pH was adjusted to 8.0 with NaOH, and the solution volume was set at 1L.
②1×TPE working solution: 10×was used for dilution.
2. Reagents for protein electrophoresis:
(1) SDS-PAGE electrophoresis buffer (10×storage solution) : The analytical balance was used to weigh 24.22 g/L Tris-base, 188 g/L glycine and 10 g/L SDS, and the magnetic stirrers were used to stir, which could be properly heated to about 40℃ to accelerate dissolution, and stored at room temperature after dissolution.
(2) 1×Tris-glycine electrophoresis solution (electrophoresis buffer working concentration) : The prepared SDS-PAGE electrophoresis buffer (10×storage solution) was diluted 1:10 with ddH2O (100 mL of 10× storage solution + 900 mL of ddH2O) to obtain a working concentration of electrophoresis buffer 1×Tris-glycine electrophoresis solution, which was stored at room temperature.
(3) 30% acrylamide: Under the condition of wearing a lab coat, mask, and gloves, 290 g/L acrylamide and 10 g/L N, N-methylene diacrylamide were weighed in the dark, and the mixture was dissolved by ddH2O in a dark condition using a magnetic stirrer, filtered through a 0.45 μm diameter filter, and stored in the dark at 4℃.
(4) Separation gel buffer Tris-HCl (1.5 mol/L pH 8.8) : 181.71 g/L Tris-base was weighed using an analytical balance, fixed volume and dissolved with ddH2O, pH was adjusted to 8.8 with HCl, and stored in a refrigerator at 4℃.
(5) Concentrated gel buffer Tris-HCl (1.0 mol/L pH 6.8) : 121.14 g/L Tris-base was weighed using an analytical balance, volume fixed and dissolved with ddH2O, pH adjusted to 6.8 with HCl, and stored in a refrigerator at 4℃.
(6) SDS-PAGE loading buffer (5×) : 25 mL 1M Tris-HCl (pH 6.8), 10g SDS, 0.5g bromophenol blue, 50 mL glycerol, and then fixed volume to 100 mL. After dispensing, 4 mL of each branch was stored at 4℃. When used, 200 ul of β-mercaptoethanol was added per 4 mL, which was stored at -20℃ for long-term use and 4℃ for short-term use. When used, it was dissolved and diluted to 2×, 1× and other required concentrations by ddH2O, and stored in the same way.
(7) 10% APS: 8 mL ddH2O was taken and dissolved by adding 1 g ammonium persulfate, and the volume was fixed to 10 mL. Filter to remove bacteria, long-term storage to -20℃ refrigerator, short-term use to 4℃, protected from light.
(8) 10% SDS: 5 g SDS was weighed using an analytical balance, dissolved in ddH2O, and quantified to 50 mL.
3. Protein electrophoresis staining and decolorization reagents:
(1) Staining solution: each 1 L solution was prepared with 450 mL methanol and 100 mL glacial acetic acid in a constant volume of ddH2O, and 2.5 g Coomassie Brilliant blue G250 was added.
(2) Decolorization solution: each 1 L solution was prepared with 450 mL of methanol, 100 mL of glacial acetic acid, and constant volume of ddH2O.
4. Protein purification related reagents
(1) Ultrasonic disinfection buffer: 20 mmol/L Tris-HCl pH 8.0, 0.5 mol/L NaCl, 1 mmol/L, 1 mg/mL lysozyme.
(2) Inclusion body washing buffer: 50 mmol/L Tris-HCl, 1 mmol/L EDTA, 50 mmol/L NaCl, 0.5% Triton X-100, pH 8.0. For use, inclusion bodies were added to this solution, washed with shaking at 37℃ for 1 to 2 h, and then centrifuged at 8000 r/min for 15 min to collect the precipitate.
(3) Inclusion body lysis buffer: 20 mmol/L Tris-HCl pH 8.0, 0.5 mol/L NaCl, 8 mol/L urea, 0.2 mmol/L DTT or 100 mmol/L β-mercaptoethanol, 2% Triton X-100.
(4) Ni2+ affinity chromatography column purification buffer: binding-buffer: the drug was weighed by analytical balance with ddH2O for constant volume, the volume was set to 1 L, and the drug concentration in solution was: Tris-base 20 mmol, NaCl 30 mmol, imidazole 10 mmol, and the pH was adjusted to 7.8 with HCl and NaOH.
Elution buffer: The binding solution formulation imidazole was changed to 30 to 60 mmol, and the pH was adjusted to 7.8.
Collection buffer: The binding solution formulation imidazole was changed to 250 mmol.
5. pH buffer preparation:
(1) Preparation of PBS reagent: the constant volume of ddH2O for drug was weighed by analytical balance to 1L (pH 7.4), and the concentration of drug in solution was as follows: After 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 2 mM KH2PO4 were filtered through 0.45 μm filter membrane, sterilized in a sterilization pot at 121℃ and cooled until use, and stored in the refrigerator at 4℃.
(2) 0.01mol/L PBS Phosphate Buffer Saline (PBS), pH7.2:
A: 0.2mol/L disodium hydrogen phosphate solution (A solution) : NaH2PO4·12H2O 35.814g, with ddH2O fixed volume to 500 mL.
B: 0.2mol/L sodium dihydrogen phosphate solution (B solution) : NaH2PO4·2H2O 15.601g, fixed volume with ddH2O to 500 mL.
36 mL of solution A, 14 mL of solution B and 8.2 g of NaCl were taken, and the volume of ddH2O was fixed to 1000 mL. The mixture was dissolved completely and packaged. After autoclaving, the mixture was stored in a refrigerator at 4℃ for later use.
(3) 1/15 mol/L Phosphate Buffer Saline (PBS) :
Solution A: 1/15 mol/L Na2HPO4 solution: 9.465g Na2HPO4, ddH2O constant volume to 1000 mL.
Solution B: l/15 mol/L Na2HPO4 solution: 9.07g Na2HPO4, ddH2O constant volume to 1000 mL.
They were stored in a brown bottle and kept in a refrigerator at 4℃. When the two liquid A and B were mixed in different proportions, the required pH buffer solution could be obtained. The mixing ratio is shown in the table below:
|
(4) Tris buffer salt solution (TBS) :
8g NaCl, 0.2g KCl and 3g Tris-base were dissolved in 800 mL of ddH2O, 0.015g phenol red was added and adjusted to pH 7.4 with HCl, and the volume was fixed to 1L with ddH2O. The aliquots were then steam sterilized at 15 psi (1.05 kg/cm2) for 20 min and stored at room temperature.
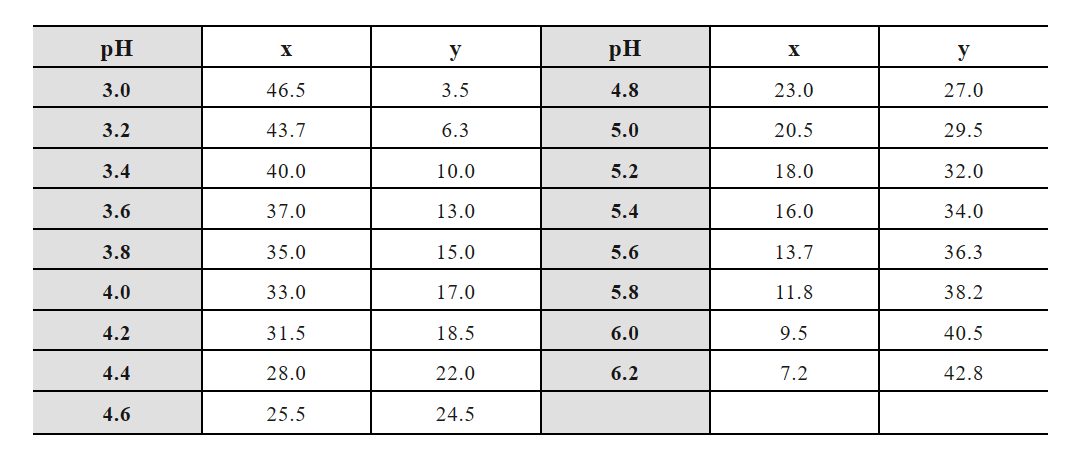
(5) Sodium carbonate-sodium bicarbonate buffer:
Storage solution A: 0.2 mol/L sodium carbonate (Na2CO3·H2O 24.8g, 1000 mL);
Storage solution B: 0.2 mol/L sodium bicarbonate (NaHCO3 16.8g, 1000 mL).
x mL A solution + y mL B solution diluted to 200 mL:
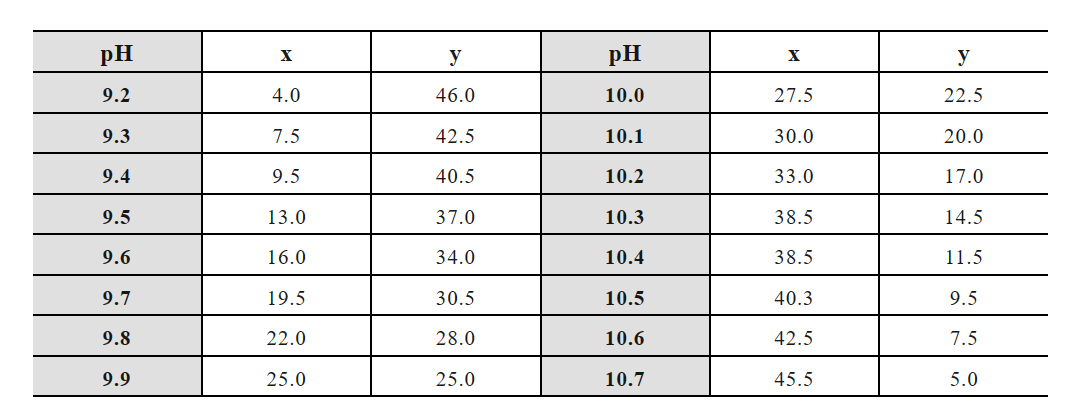
(6) Sodium dihydrogen phosphate-disodium hydrogen phosphate buffer:
Storage solution A: 0.2 mol/L sodium dihydrogen phosphate (NaH2PO4·H2O 27.6g or NaH2PO4·2H2O 31.21g, 1000 mL);
Storage solution B: 0.2 mol/L disodium hydrogen phosphate (NaH2PO4·2H2O 35.61g or NaH2PO4·12H2O 71.64g, 1000 mL).
x mL A solution + y mL B solution diluted to 100 mL:
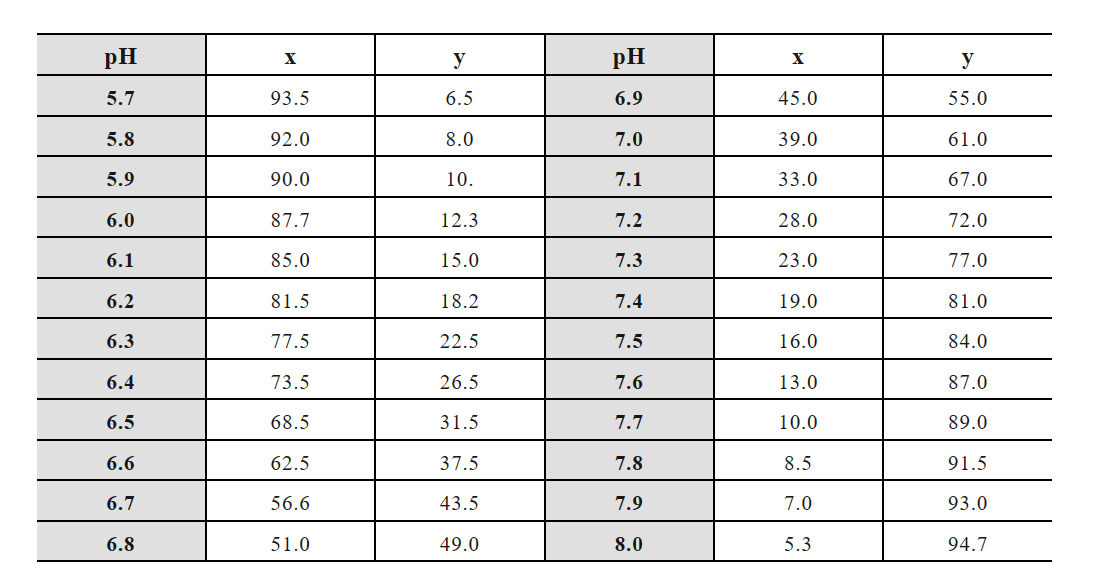
(7) Tris-HCl buffer
Storage solution A: 0.2 mol/L Tris (C4H11NO3, 24.2g, 1000 mL);
Storage solution B: 0.2 mol/L hydrochloric acid (concentrated HCl 17.1 mL was prepared into 1000 mL).
50 mL of solution A + x mL of solution B diluted to 200 mL:

(8) Citric acid-sodium citrate buffer:
Storage solution A: 0.1 mol/L citric acid (C6H8O7, 19.21g, 1000 mL);
Storage solution B: 0.1 mol/L sodium citrate (C6H5O7Na3·2H2O 29.41g, 1000 mL).
x mL A solution + y mL B solution diluted to 100 mL:
(9) Acetate-sodium acetate buffer:
Storage solution A: 0.2 mol/L acetic acid (11.55 mL glacial acetic acid diluted to 1000 mL);
Storage solution B: 0.2 mol/L sodium acetate (16.4 g of C2H2O2Na or 27g of C2H2O2Na·3H2O, 1000 mL).
x mL A solution + y mL B solution diluted to 1000 mL:

6. Cell culture and cell fusion solution:
1. 0.25% trypsin-0.02% EDTA mixed digest:
First, 0.25g of Trypsin powder was dissolved with a small amount of 0.01mol/L PBS, and then 20.0 mg EDTA powder and the remaining liquid were fixed to 100 mL, placed in a water bath at 37℃ for about 1 hour (until the liquid was completely dissolved and transparent), filtered, and stored in a refrigerator at 4℃.
2. Hanks solution:
(1) Stock solution
① Stock solution A: NaCl 160g + KCl 8g + MgSO4·7H2O 2g + MgCl2·6H2O 2g dissolved in 800 mL ddH2O.
2.8 g of CaCl2(anhydrous) was dissolved in 100 mL of ddH2O.
After mixing the two liquids, add water to 1000 mL and filter with filter paper, then add 2 mL chloroform for preservative, and store in the refrigerator at 4℃ for later use.
② Stock solution B: Na2HPO4·12H2O 3.04 g + KH2PO4 1.2g + glucose 20.0g
It was dissolved in 800 mL of ddH2O, filtered with filter paper, then 80 mL of 0.5% phenol red was added, and then water was added to 1000 mL. Finally, 2 mL chloroform was added to antiseptic and stored in a refrigerator at 4℃ for later use.
(2) Use liquid
One portion of AB solution and 18 portion of ddH2O solution were mixed, packaged, and wrapped. After autoclaving, the bottles were stored in a refrigerator at 4℃. 5.6% NaHCO3 was used to adjust the pH value to the required requirements.
3. LB medium (Luria-Bertani medium) :
(1)Liquid LB: LB liquid medium was prepared in a 500 mL conical flask for cell culture and protein induction, and the drug was weighed using an analytical balance: 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl were added to a conical flask for constant volume, sterilized in ddH2O, programmed at 121℃ for 15 min, and cooled to room temperature.
(2)Solid LB: Solid LB medium was prepared in conical flasks for cell culture, streaking and resistance screening. The drugs were first weighed with an analytical balance, including 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, and 15 g/L AGAR powder, sterilized in a high-pressure water bath at 121℃ for 15 min, cooled to about 50℃, and then mixed with resistance. The as-yet unset LB solid medium was poured into the Petri dish at approximately 25 mL/ plate and cooled until completely set. Subsequently, the graft membrane was used for sealing and inverted to be used.
4. IPTG: isopropyl-β-D-thiogalactoside(isopropyl-β-D-thiogalactoside), as a protein inducer. The mother solution was 1 M/L, and the working solution was 1 mM/L.
7. Common antibiotics:
Antibiotics are commonly added when performing microbial cultures in the laboratory to prevent bacterial contamination or for specific types of strain screening. Here are the storage and administration concentrations of some commonly used antibiotics:
A+: Stock: 100 mg/mL; Act: 50 μg/mL
K+ Sulfate: Stock: 50 mg/mL; Act: 50 μg/mL
Gentamycin: Stock: 50 mg/mL; Act: 50 μg/mL
Rifampicin: Stock: 50 mg/mL; Act: 50 μg/mL
Carbenicillin sodium: Stock: 50 mg/mL; Act: 50 μg/mL
Chloramphenicol: Stock: 34 mg/mL (dissolved in ethanol); Act: 25 μg/mL (tight plasmid) to 170 μg/mL (loose plasmid)
Streptomycin: Stock: 10 mg/mL; Act: 10 μg/mL (tight plasmid)
To 50 μg/mL (relaxed plasmid)
Tetra+: Stock: 5 mg/mL (dissolved in ethanol); Act: 10 μg/mL (tight plasmid) to 50 μg/mL (loose plasmid)
It should be noted that the concentration of antibiotics used may be adjusted according to the specific needs of the experiment and the characteristics of the strain. In addition, antibiotics are usually stored at -20°C and should be stored in light-tight containers to prevent light-induced degradation. When using antibiotics, it should also be noted that their dissolution media, for example Chloramphenicol and Tetra+ are usually dissolved in ethanol, while some other antibiotics such as A+ and K+ are dissolved in water.
Aladdin:https://www.aladdinsci.com/