Fluid chemistry: Lighting the fire of hope for the large-scale production of anticancer drugs

The latest research: The anticancer drug Divarasib (GDC-6036) quinazoline organic zinc achieves multi-kilogram scale production
High functional quinazoline organic zinc of KRAS G12C inhibitor Divarasib (GDC-6036) was synthesized by continuous flow chemistry
A paper published August 24 at Org. Process Res. Dev by Professor Sean M. Kell et al. describes a robust and efficient continuous flow process technique for the synthesis of quinazoline organozinc reagents for KRAS G12C inhibitor divarasib. By using a continuous flow reactor system, the researchers overcame many of the challenges of traditional batch metallization processes, successfully transiting from initial laboratory proof-of-concept experiments to multi-kilogram scale production and achieving excellent yields and performance in subsequent selective Negishi cross-coupling reactions.
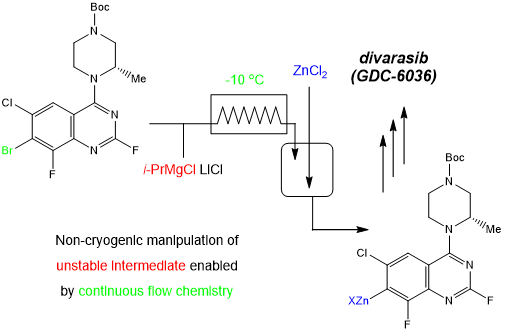
About Divarasib
Divarasib is a highly selective covalent inhibitor of KRAS G12C with oral bioavailability and is currently in advanced clinical development for the treatment of metastatic non-small cell lung cancer.
A new generation of KRAS inhibitors has demonstrated good clinical activity and a controllable safety profile. Of the 29 patients reported, 66% had an unconfirmed overall response and 62% had a confirmed overall response. This means that more than 60 percent of advanced patients had their tumors shrink by more than 30 percent. With nearly 70% of patients experiencing significant tumour shrinkage, divarasib offers hope for bowel cancer patients.
Divarasib's amplification process route
The recently discovered heterotopic inhibitors of KRAS covalent bonds provide an exciting opportunity to regulate this important target, and traditional small molecule inhibitors were once considered "unmedicable." The development of robust and scalable new process scale-up routes for these structurally complex molecules is therefore critical for ongoing clinical trials.
The continuous flow process uses quinazoline organic zinc reagent as the key intermediate, and through the innovative amplification reaction technology and the design of the plug flow reactor, the problems of instability and reactor plugging of quinazoline organic magnesium are successfully solved. First, through laboratory-scale studies, the researchers determined the optimal reaction conditions and process parameters. Subsequently, a reactor group consisting of PFR and CSTR was designed to control temperature and residence time.
The key quinazoline organozinc coupling agent 3 in the initial batch was obtained by a metal halogen exchange reaction between Bromoquinazoline 1 and i-PrMgCl-LiCl at -70 oC to form quinazoline organomagnesium intermediate 2, which was then demetallized with ZnCl2 solution before heating to 10 oC (Scheme 1).
Although the process has been successfully scaled to 125 kg, the reaction must be carried out in a dedicated reactor group with limited capacity, which greatly limits the yield and further scale-up of the batch process. In addition, the process is expected to consume excessive amounts of liquid nitrogen, have a significant impact on the environment, and require significant logistical work.
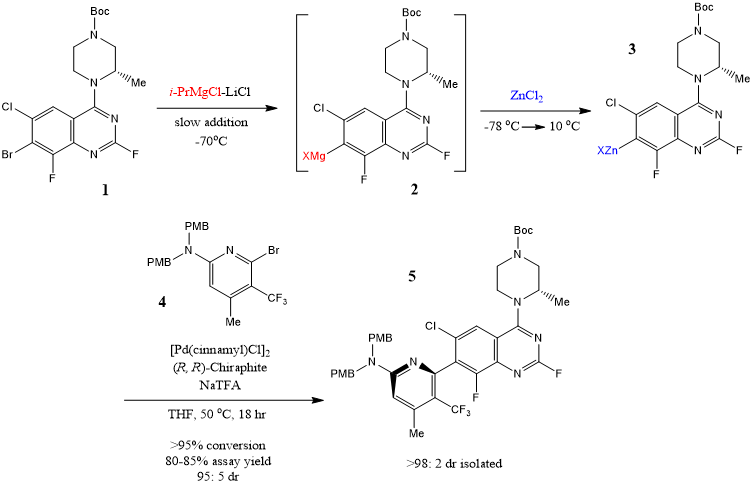
Scheme 1. Second-Generation Atroposelective Negishi Cross-Coupling
Since the optimization of the continuous flow process leads to high material consumption during the research phase, the existing batch reaction conditions are first studied to gain important knowledge about the basic reaction dynamics, and then the parameters are optimized in a dedicated plug flow reactor system.

|
Table 1. Quinazoline Organomagnesium Stability at 0℃
To validate the understanding of the process and confirm scale-up performance from laboratory development to plant scale, the researchers designed and installed an intermediate test reactor that operated at a scale closer to the projected plant throughput (1.27 kg/h).
Crude organic solutions containing zinc intermediates 3 were continuously root-shore coupled at 20 g (42 mmol) and 1000 g (2.09 mol) scales in a classical intermittent mode without further treatment.
|
Table 2. Ramp Up and Steady State Performance
In laboratory tests and pilot activities, this continuous flow process has demonstrated excellent stability and scalability. The stable and high purity quinazoline organic zinc reagent was synthesized by precise temperature and residence time control. The experimental results show that there is no obvious deviation of process parameters in the large-scale test, and the conversion rate and purity can maintain good.
Brief Summary
This study provides a scalable continuous flow process for the synthesis of high functionalized quinazoline organic zinc from KRAS G12C inhibitor divarasib. The process is expected to drive the development and industrial production of more drugs, removing a major logistical hurdle for scaling up highly sensitive reaction sequences. At the same time, divarasib's research brings new hope to cancer patients and is another major milestone in the fight against cancer.
Aladdin:https://www.aladdinsci.com |
List of related products

