How to find the right secondary antibody

Secondary antibodies bind primary antibodies to allow detection, sorting and purification of target antigens. Since they are specific for the primary antibody species and isoform, they can detect the protein of interest. The need for a secondary antibody depends on your antibody detection method.
Direct and indirect detection methods
The method of detecting the target antigen can be direct or indirect (Figure 1):
Direct: detection of the antigen by means of a primary antibody coupled directly to the marker (i.e. coupled primary antibody), thus eliminating the need for a secondary antibody.
Indirect: The antigen is detected by a conjugated secondary antibody, which is produced against the host species of the primary antibody and binds to it. The indirect approach provides higher signal intensity. This is because multiple approaches to labeling the secondary antibody can bind each antigen indirectly (Figure 1). Indirect assays may also include an amplification step to increase signal intensity.
The choice of direct or indirect assay usually depends on the expression level of the target antigen. Direct assays are suitable for analyzing highly expressed antigens. Conversely, indirect assays are more suitable for studying poorly expressed antigens, which benefit from the signal amplification provided by the secondary antibody.
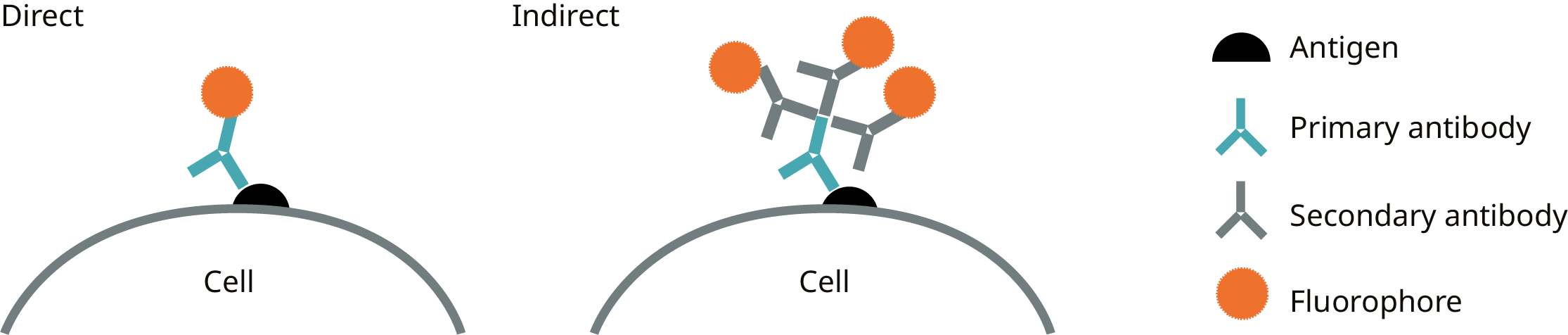
Fig. 1. Schematic representation of direct and indirect detection of antigens using fluorophore-labelled antibodies.
Both methods have some points and limitations to consider before choosing the most appropriate method for your experiment (Table 1).
Table 1.Comparison of direct and indirect detection methods.
|
Choosing a directly conjugated primary antibody
The use of directly conjugated primary antibodies (e.g., conjugated to enzyme markers or fluorescent markers) can speed up and simplify protocols by eliminating the secondary antibody staining step. In addition, conjugated primary antibodies minimize species cross-reactivity and eliminate non-specific binding that may occur with secondary antibodies. Fluorescent Conjugated Primary Antibodies are ideal for multicolour experiments as they give you the flexibility to assemble the required multiplex panels.
When choosing the primary antibody conjugate, pay attention to the specificity of the antibody. It is best to choose recombinant monoclonal antibodies, which have high specificity and good batch-to-batch consistency.
Choosing a suitable secondary antibody
If an indirect assay is used, a suitable secondary antibody needs to be selected.
Secondary antibodies are produced by immunising an animal with an antibody that acts as an immunogen. The secondary antibody produced will bind to the type of antibody the animal has been immunised with.
The descriptive name of the secondary antibody reveals the type of primary antibody to which it binds. These names include the prefix "anti-" which indicates their reactivity. For example, if an animal is immunised with rabbit IgG, the secondary antibody produced will bind to rabbit IgG and is called anti-rabbit IgG.
When choosing a secondary antibody, you need to consider whether it will selectively bind to the primary antibody and enable you to detect the antigen, depending on several key factors.
Host species
The host species used to culture the secondary antibody must be different from the primary antibody. For example, if the primary antibody is cultured in rabbits, the secondary antibody would need to be cultured in another species; a donkey-anti-rabbit secondary antibody would be appropriate.
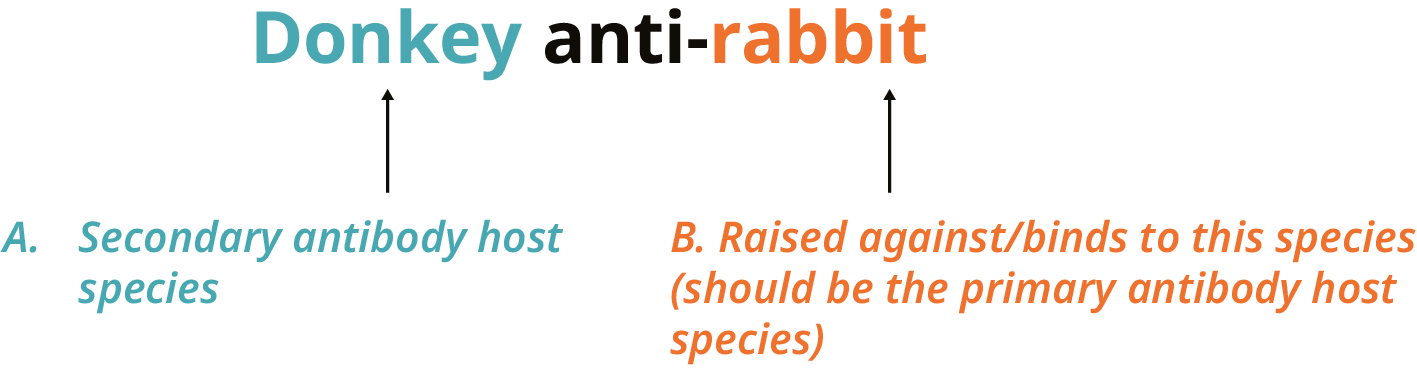
Figure 2. Use the name of the secondary antibody to find out which species it reacts with. The secondary antibody was cultured in donkeys (A) and bound to rabbit antibodies (B).
Combining isotype and specificity
The secondary antibody must bind to the isotype of the primary antibody.
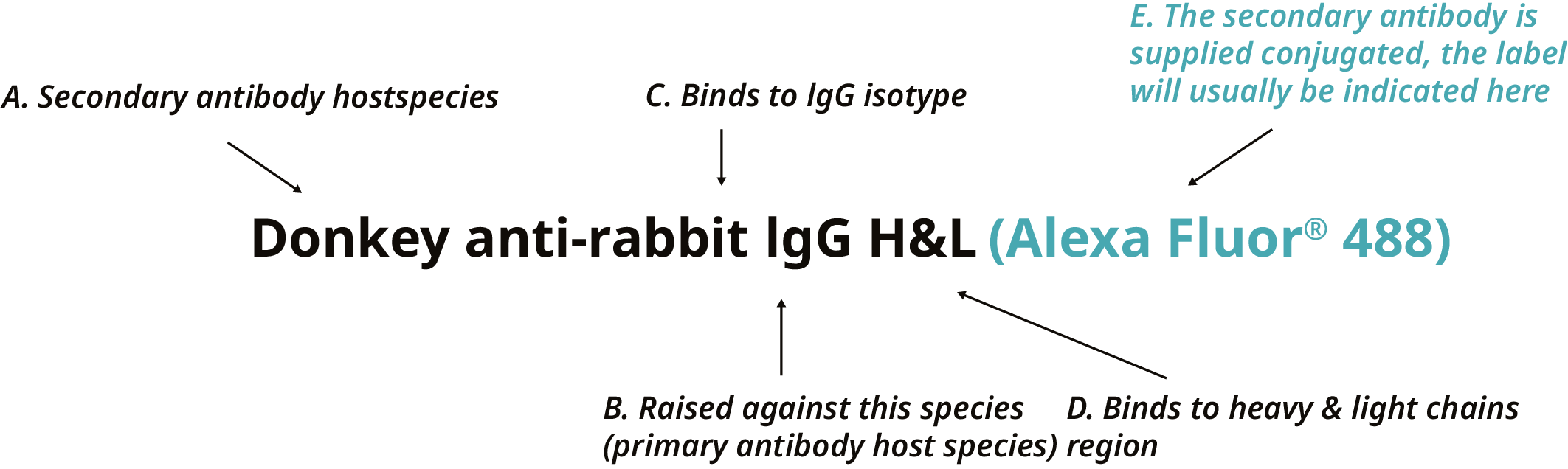
The primary antibody is usually of IgG isotype. Therefore, the secondary antibody needs to bind to IgG. Typically, anti-IgG secondary antibodies bind to heavy and light chains (H&L), but can also bind to other regions of the primary antibody.

Figure 3. Utilising the name of a secondary antibody to comprehend the species, isotype, and region of the primary antibody to which it binds. This secondary antibody binds exclusively to the H&L region of rabbit IgGs (B, C, D).
Conjugate selection for different applications
The presence of target proteins can be observed by combining labels such as fluorescent dyes, proteins, enzymes and biotin with secondary antibodies.
Fluorescent labels emit a visible range of light when excited by a certain wavelength of light. There are several types of fluorescent labels, all with their own excitation and emission characteristics.
Enzyme markers, such as horseradish peroxidase (HRP) and alkaline phosphatase (AP), form coloured precipitates when combined with appropriate substrates.
Biotinylated antibodies can be used for signal amplification when conjugated to an avermectin-biotin-enzyme or fluorescent dye complex (often abbreviated to ABC reagent), i.e. avermectin or streptavidin conjugated to an enzyme or fluorescent dye.
Figure 4. The secondary antibody label is usually placed at the end of the antibody name. This secondary antibody is linked to the green fluorophore Alexa Fluor® 488.
The choice of conjugate depends on the application. Enzyme-conjugated secondary antibodies are most popular for ELISA or Western blotting applications. In contrast, secondary antibodies conjugated to fluorescent proteins or dyes (e.g. Alexa Fluor®) are preferred in flow cytometry and ICC.
Below we will list some suggested secondary antibodies for the main applications you might use them for (Table 2).
Table 2. Selection of secondary antibodies labelled with enzymes or fluorochromes for different applications.
|
IHC = immunohistochemistry, ICC = immunocytochemistry, FACS = fluorescence-activated cell sorting, HRP = horseradish peroxidase, AP = alkaline phosphatase
Reference
1. https://ptglab.com/news/blog/secondary-antibody-selection/ Secondary antibody selection guide
2."Secondary Antibodies as Probes". www.thermofisher.com. Retrieved 2017-05-31.
3."F(ab')₂ Fragment Secondary Antibodies - Jackson ImmunoResearch". www.jacksonimmuno.com. Retrieved 2021-01-29.
4."Fab Fragment Secondary Antibodies - Jackson ImmunoResearch". www.jacksonimmuno.com. Retrieved 2021-01-29.
5."Secondary antibody review based on formal publications". secondary-antibody.com. Archived from the original on 2008-04-17. Retrieved 2008-04-16.