Indium (low valent)
Product Manager
Sandra Forbes
Low-valent metal compounds are frequently employed as reducing agents. Given that its most stable oxidation state is 3+, indium is utilized in its 2+ oxidation state to function as a one-electron reducing agent. Hydrides of indium, like InCl2H, which can be synthesized using readily accessible Et3SiH or NaBH4 and InCl3, provide gentle reaction conditions and exhibit low toxicity. Consequently, they represent promising substitutes for Bu3SnH.
Recent Literature

A diastereoselective reductive aldol reaction, facilitated by Et3SiH and catalyzed by InBr3, has been devised. This three-component system exclusively yields silyl aldolates as products, without any accompanying side reactions.
I. Shibata, H. Kato, T. Ishida, M. Yasuda, A. Baba, Angew. Chem. Int. Ed., 2004, 43, 711-714.
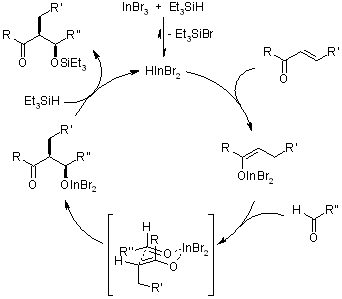

The oxidative addition of In/InCl3 to enones exclusively occurs in aqueous environments, resulting in the formation of water-tolerant, ketone-based indium homoenolates. The practical application of these indium homoenolates was showcased through the synthesis of 1,4-dicarbonyl compounds, achieved via palladium-catalyzed coupling reactions with acid chlorides.
Z.-L. Shen, K. K. K. Goh, H.-L. Cheong, C. H. A. Wong, Y.-C. Lai, Y.-S. Yang, T.-P. Loh, J. Am. Chem. Soc., 2010, 132, 15852-15855.
DOI: 10.1021/ja106925f
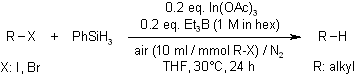
When bromo- and iodoalkanes react with PhSiH3 in THF at 70°C, catalyzed by In(OAc)3 and in the presence of Et3B and air, dehalogenated alkanes are obtained in good to high yields. The use of 2,6-lutidine as an additive facilitates the efficient reduction of both simple and functionalized iodoalkanes in EtOH. Additionally, GaCl3 has been identified as an effective catalyst for the reduction of haloalkanes using poly(methylhydrosiloxane).
K. Miura, M. Tomita, Y. Yamada, A. Hosomi, J. Org. Chem., 2007, 72, 787-792.
DOI: 10.1021/jo061880o
The direct reduction of alcohols to their corresponding alkanes, employing chlorodiphenylsilane as the hydride source and a catalytic amount of InCl3, exhibits high chemoselectivity towards benzylic, secondary, and tertiary alcohols. Notably, this method does not reduce primary alcohols or functional groups that are typically susceptible to reduction by standard methods, such as esters, chloro, bromo, and nitro groups.
M. Yasuda, Y. Onishi, M. Ueba, T. Miyai, A. Baba, J. Org. Chem., 2001, 7741-7744.
DOI: 10.1021/jo0158534
Using indium tri(isopropoxide) as a catalyst, the Meerwein-Ponndorf-Verley reduction of aliphatic and aromatic aldehydes in 2-propanol selectively yields the corresponding primary alcohols in good to excellent yields at room temperature. This reaction is compatible with a diverse array of functional groups, including alkenes, ethers, ketones, esters, nitriles, and nitros.
J. Lee, T. Ryu, S. Park, P. H. Lee, J. Org. Chem., 2012, 77, 4821-4825.
DOI: 10.1021/jo300236u
In the presence of indium bromide (InBr3) as a catalyst, the combination of 1,1,3,3-tetramethyldisiloxane (TMDS) and trimethylbromosilane (Me3SiBr) facilitated a direct bromination of carboxylic acids. This reducing system demonstrated tolerance to various functional groups and yielded the corresponding alkyl bromides in excellent quantities.
T. Moriya, S. Yoneda, K. Kawana, R. Ikeda, T. Konakahara, N. Sakai, Org. Lett., 2012, 14, 4842-4845.
DOI: 10.1021/ol302168q
A highly efficient indium(III)-catalyzed reductive bromination or iodination of various carboxylic acids, utilizing 1,1,3,3-tetramethyldisiloxane (TMDS) and a halogen source, displays compatibility with numerous functional groups. This indium-based catalytic system is also effective for the reductive iodination of aldehydes, acyl chlorides, and esters. Additionally, this reducing system enables the one-pot synthesis of alkyl halides and amine derivatives.
T. Moriya, S. Yoneda, K. Kawana, R. Ikeda, T. Konakahara, N. Sakai, J. Org. Chem., 2013, 78, 10642-10650.
DOI: 10.1021/jo401529j
Using indium(III)-catalyzed reductive iodination or bromination of carboxylic acids, a one-pot synthesis of alkyl cyanides can be achieved directly from carboxylic acids, via intermediate alkyl iodides or alkyl bromides.
T. Moriya, K. Shoji, S. Yoneda, R. Ikeda, T. Konakahara, N. Sakai, Synthesis, 2013, 45, 3233-3238.
We report a novel one-pot method for the direct reductive transformation of esters into their corresponding ethers using Et3SiH, in the presence of a catalytic amount of InBr3. This straightforward catalytic system demonstrates remarkable tolerance towards a variety of functional groups.
N. Sakai, T. Moriya, T. Konakahara, J. Org. Chem., 2007, 72, 5920-5922.
DOI: 10.1021/jo070814z
Indium-Catalyzed Henry-Type Reaction of Aldehydes with Bromonitroalkanes
R. G. Soengas, A. M. S. Silva, Synlett, 2012, 23, 873-876.
Indium(0) facilitates an efficient synthesis of benzylic hydroperoxides in excellent yields from a diverse range of benzyl bromides under aerobic conditions at room temperature. Furthermore, it has been demonstrated to mediate a tandem hydroperoxidation-Michael addition reaction with (E)-1-(bromomethyl)-2-(2-nitrovinyl)benzene.
Y. Hou, J. Hu, R. Xu, S. Pan, X. Zeng, G. Zhong, Org. Lett., 2019, 21, 4428-4432.
DOI: 10.1021/acs.orglett.9b01070
Indium hydride (Cl2InH) was synthesized through the transmetalation of InCl3 with Et3SiH. In contrast to the previously reported system involving NaBH4 and InCl3, where the coexistence of borane could lead to unwanted side reactions, the use of Et3SiH instead of NaBH4 enables efficient hydroindation of alkynes.
N. Hayashi, I. Shibata, A. Baba, Org. Lett., 2004, 6, 4981-4983.
DOI: 10.1021/ol047849v
N. Hayashi, I. Shibata, A. Baba, Org. Lett., 2004, 6, 4981-4983.
DOI: 10.1021/ol047849v
O. N. Hayashi, I. Shibata, A. Baba, Org. Lett., 2004, 6, 4981-4983.
DOI: 10.1021/ol047849v
Employing indium metal in an aqueous ethanol solution facilitates a highly selective reduction of aryl propargyl ethers, amines, and esters, yielding high productivities. This approach circumvents over-reduction of the newly formed double bond and demonstrates compatibility with various easily reducible functionalities.
B. C. Ranu, J. Dutta, S. K. Guchhait, J. Org. Chem., 2001, 66, 5413-5418.
DOI: 10.1021/jo010262z
Nickel-catalyzed asymmetric hydrogenation of α,β-unsaturated esters, nitriles, ketones, and allylic alcohols proceeds with high enantioselectivity when utilizing acetic acid or water as the hydrogen source, in conjunction with indium powder serving as an electron donor. Additionally, asymmetric deuteration of α,β-unsaturated esters can be accomplished using deuterated water.
S. Guo, X. Wang, J. S. Zhou, Org. Lett., 2020, 22, 1204-1207.
DOI: 10.1021/acs.orglett.0c00112
The in situ generation of dichloroindium hydride (Cl2InH) from a catalytic amount of indium(III) chloride and sodium borohydride in acetonitrile facilitates the reduction of activated vic-dibromides to their corresponding (E)-alkenes in exceptional yields.
B. C. Ranu, A. Das, A. Hajira, Synthesis, 2003, 1012-1014.
DOI: 10.1055/s-2003-39165
B. C. Ranu, A. Das, A. Hajira, Synthesis, 2003, 1012-1014.
DOI: 10.1055/s-2003-39165
Indium- and zinc-mediated dehalogenation reactions of vicinal dihalides in an aqueous solvent provide a straightforward and gentle method for synthesizing various allenylmethyl aryl ethers and monosubstituted allenes in excellent yields.
M.-H. Lin, W.-S. Tsai, L.-Z. Lin, S.-F. Hung, T.-H. Chuang, Y.-J. Su, J. Org. Chem., 2011, 76, 8518-8523.
DOI: 10.1021/jo2015104
The in situ generation of acylindium reagents from readily available acid chlorides and indium, followed by a Pd-catalyzed cross-coupling reaction with aryl iodides, triflates, and alkenyl triflates, enables the reversed-polarity synthesis of various unsymmetric aryl-aryl and alkenyl-aryl ketones.
D. Lee, T. Ryu, Y. Park, P. H. Lee, Org. Lett., 2014, 16, 1144-1147.
DOI: 10.1021/ol500003g
A novel, convenient, and stereoselective synthesis of trisubstituted E-alkenones has been accomplished through an InCl3-mediated chemoselective reduction of Baylis-Hillman adducts, utilizing NaBH4 as the reductant.
B. Das, J. Banerjee, N. Chowdhury, A. Majhi, H. Holla, Synlett, 2006, 1879-1882.
The use of Et3SiH leads to fewer side reactions in the intramolecular cyclization of enynes when compared to the NaBH4-InCl3 system reported previously.
N. Hayashi, I. Shibata, A. Baba, Org. Lett., 2004, 6, 4981-4983.
DOI: 10.1021/ol047849v
A reaction between carbonyl compounds and chlorodimethylsilane, catalyzed by indium(III) hydroxide, yields the corresponding deoxygenated chlorination products. During the reaction, ester, nitro, cyano, or halogen groups remain unaffected. In contrast, typical Lewis acids like TiCl4, AlCl3, and BF3·OEt2 exhibited no catalytic activity. The reaction mechanism is explored and discussed.
Y. Onishi, D. Ogawa, M. Yasuda, A. Baba, J. Am. Chem. Soc., 2002, 124, 13690-13691.
DOI: 10.1021/ja0283246
Under very mild conditions, organic azides undergo easy and chemoselective reduction to their corresponding amines through reaction with dichloroindium hydride. Notably, γ-azidonitriles undergo exceptional cyclization to yield pyrrolidin-2-imines.
L. Benati, G. Bencivenni, R. Leardini, D. Nanni, M. Minozzi, P. Spagnolo, R. Scialpi, G. Zanardi, Org. Lett., 2006,8, 2499-2502.
DOI: 10.1021/ol0606637
L. Benati, G. Bencivenni, R. Leardini, D. Nanni, M. Minozzi, P. Spagnolo, R. Scialpi, G. Zanardi, Org. Lett., 2006, 8, 2499-2502.
DOI: 10.1021/ol0606637
In the presence of indium and dilute aqueous HCl, a three-component reaction involving nitro compounds, carbonyl compounds, and phosphites facilitates the high-yielding synthesis of α-amino phosphonates at room temperature. This one-pot process encompasses the reduction of nitro compounds to amines, the formation of imines, and subsequent hydrophosphonylation.
B. Das, G. Satyalakshmi, K. Suneel, K. Damodar, J. Org. Chem., 2009, 74, 8400-8402.
DOI: 10.1021/jo901765s
An efficient synthetic approach yields tri- and tetra-substituted allenes through the reaction of allylindium reagents, which are generated in situ from indium and allyl bromides, with 3°-propargyl alcohols.
K. Lee, P. H. Lee, Org. Lett., 2008, 10, 2441-2444.
DOI: 10.1021/ol800719g
When various carbonyl compounds react with organoindium reagents, which are generated in situ from indium and 1-bromopent-4-en-2-yne derivatives, functionalized vinyl allenols are obtained in good yields. Subsequent treatment of these vinyl allenols with a gold catalyst, dienophile, or indium trihalide results in the production of functionalized dihydrofurans, cyclohexenes, or 2-halo-1,3-diene derivatives in very good yields.
J. Park, S. Hong, P. H. Lee, Org. Lett., 2008, 10, 5067-5070.
DOI: 10.1021/ol802073q
Using organoindium reagents generated in situ from indium and ethyl 4-bromo-2-alkynoates, Pd-catalyzed cross-coupling reactions of both electron-rich and electron-poor aryl iodides selectively produced ethyl 2-aryl-2,3-alkadienoates in good yields.
P. H. Lee, J. Mo, D. Kang, D. Eom, C. Park, C.-H. Lee, Y M. Jung, H. Hwang, J. Org. Chem., 2011, 76, 312-315.
DOI: 10.1021/jo1020085
At room temperature, a system of indium and pivaloyl chloride was employed to deoxygenate sulfoxides and aza-aromatic N-oxides, yielding the corresponding sulfides and aza-aromatics in high yields.
E. S. Park, S. Hwan, Lee, J. H. Lee, H. J. Rhee, C. M. Yoon, Synthesis, 2005, 3499-3501.
E. S. Park, S. Hwan, Lee, J. H. Lee, H. J. Rhee, C. M. Yoon, Synthesis, 2005, 3499-3501.
Quoted from:
https://www.organic-chemistry.org/chemicals/reductions/indiumlowvalent.shtm
Aladdinsci: https://www.aladdinsci.com




























