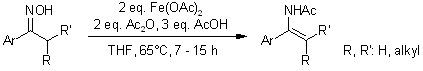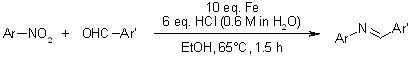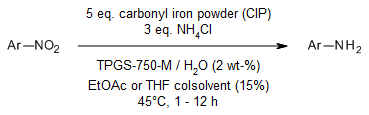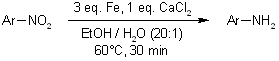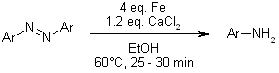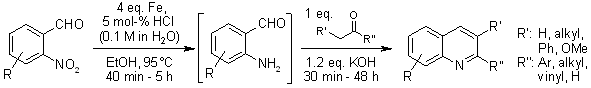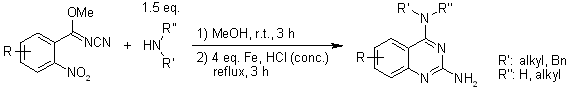Iron (low valent)
Sandra Forbes
Product Manager

Recent Literature

Fe(0) serves as a cost-effective and environmental friendly substitute for Cr(II) in the olefination of carbonyls with activated polyhalides. It has shown compatibility with a wide range of functional groups, such as unprotected phenols, aryl nitro compounds, carboxylic acids, and alkyl nitriles.
J. R. Falck, R. Bejot, D. K. Barma, A. Bandyopadhyay, S. Joseph, C. Mioskowski, J. Org. Chem., 2006, 71, 8178-8182.
DOI: 10.1021/jo061445u

A simple and highly efficient Reformatsky reaction of aldehydes has been conducted in THF using low-valent iron or copper, which are generated in situ through a bimetal redox strategy involving the reduction of Fe(III) or Cu(II) salts with magnesium.
A. Chattopadhyay, A. Kr. Dubey, J. Org. Chem., 2007, 72, 9357-9359.
DOI: 10.1021/jo0710984

An efficient, affordable, and practical Reformatsky reaction between α-halo esters and carbonyl compounds is enabled by low-cost iron(0) powder. Using a catalytic amount of iodine, the reactions successfully produced synthetically valuable β-hydroxyl carbonyl compounds in good yields.
X.-Y. Liu, X.-R. Li, C. Zhang X.-Q. Chu, W. Rao, T.-P. Loh, Z.-L. Shen, Org. Lett., 2019, 21, 5873-5878.
DOI: 10.1021/acs.orglett.9b01999
An iron complex with electronically coupled acidic and hydridic hydrogens effectively catalyzes the hydrogenation of ketones under mild conditions, showing strong chemoselectivity for aldehydes, ketones, and imines. Importantly, isolated carbon-carbon double and triple bonds, aryl halides, nitrates, epoxides, and ester groups are not affected by these hydrogenation conditions.
C. P. Casey, H. Guan, J. Am. Chem. Soc., 2007, 129, 5816-5817.
DOI: 10.1021/ja071159f
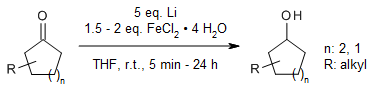
To achieve highly stereoselective reductions of many five- and six-membered cyclic ketones into their most thermodynamically stable alcohols, the ketones are reacted with lithium dispersion and either FeCl₂·4H₂O or CuCl₂·2H₂O in THF at room temperature. This method is more convenient and efficient compared to other commonly used protocols for similar reductions.
N. Kennedy, T. Cohen, J. Org. Chem., 2015, 80, 8134-8141.
A simple and practical approach for synthesizing N-acetyl α-arylenamides from the corresponding ketoximes using ferrous acetate as the reducing agent provides mild reaction conditions, straightforward purification steps, and high yields for a range of N-acetyl enamides.
W. Tang, A. Capacci, M. Sarvestani, X. Wei, N. K. Yee, C. H. Senanayake, J. Org. Chem., 2009, 74, 9528-9530.
DOI: 10.1021/jo902259u
An intermolecular reductive Schiff base formation from nitroarenes and benzaldehydes to produce diarylimines is conducted using iron powder and dilute acid. This method accommodates a variety of functional groups and typically proceeds quantitatively, often eliminating the need for purification.
A. L. Korich, T. S. Hughes, Synlett, 2007, 2602-2604.
The extremely affordable carbonyl iron powder (CIP), a highly active commercial-grade iron powder, facilitates a particularly mild, safe, efficient, and environmentally friendly reduction of aromatic and heteroaromatic nitro groups in water. These reductions are performed in a recyclable aqueous medium with nanomicelles made from TPGS-750-M.
N. R. Lee, A. A. Bikovtseva, M. Cortes-Clerget, F. Gallou, B. H. Lipshutz, Org. Lett., 2017, 19, 6518-6521.
DOI: 10.1021/acs.orglett.7b03216
An effective Fe/CaCl₂ system allows for the reduction of nitroarenes and reductive cleavage of azo compounds through catalytic transfer hydrogenation, accommodating sensitive functional groups such as halides, carbonyls, aldehydes, acetyls, nitriles, and esters with excellent yields. The straightforward experimental procedure and easy purification make this protocol particularly beneficial.
S. Chandrappa, T. Vinaya, T. Ramakrishnappa, K. S. Rangappa, Synlett, 2010, 3019-3022.
An effective Fe/CaCl₂ system facilitates the reduction of nitroarenes and the reductive cleavage of azo compounds through catalytic transfer hydrogenation, accommodating sensitive functional groups such as halides, carbonyls, aldehydes, acetyls, nitriles, and esters with outstanding yields. The straightforward experimental procedure and simple purification process enhance the advantages of this protocol.
S. Chandrappa, T. Vinaya, T. Ramakrishnappa, K. S. Rangappa, Synlett, 2010, 3019-3022.
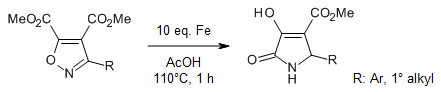
A heterocycle-heterocycle interconversion strategy yields 4,5-disubstituted 3-hydroxy-2-pyrrolidinones in good yields. The described reductive rearrangement method particularly enables the synthesis of unsubstituted 3-hydroxy-2-pyrrolidinone at the nitrogen position for further functionalization.
P. Kamath, V. Jadhav, M. Lal, Synlett, 2021, 32, 1146-1150.
DOI: 10.1055/a-1492-8216
The reduction of o-nitroarylcarbaldehydes to o-aminoarylcarbaldehydes using iron with a catalytic amount of aqueous hydrochloric acid, followed by in situ condensation of the resulting amines with ketones or aldehydes (Friedlaender quinoline synthesis), produces mono- or disubstituted quinolines in very good yields.
A.-H. Li, D. J. Beard, H. Coate, A. Honda, M. Kadablbajoo, A. Kleinberg, R. Laufer, K. M. Mulvihill, A. Nigro, P. Rastogi, M. W. Siu, A. G. Steinig, T. Wang, D. Werner, A. P. Crew, M. J. Mulvihill, Synthesis, 2010, 1629-1632.
A tandem condensation of a cyanoimidate with an amine, followed by reductive cyclization in an iron-HCl system, provides an efficient pathway to N4-substituted 2,4-diaminoquinazolines. An extra N-alkylation step can yield two fused heterocycles in a one-pot process.
P. Yin, N. Liu, Y.-X. Deng, Y. Chen, Y. Deng, L. He, J. Org. Chem., 2012, 77, 2649-2658.
DOI: 10.1021/jo2023697
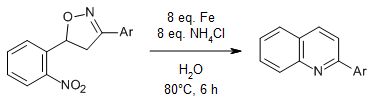
Quinolines can be produced from Δ2-isoxazolines under reductive conditions. This reductive cyclization to quinolines occurs with the use of iron or sodium dithionite in metal-free conditions.
P. Kamath, R. C. Viner, S. C. Smith, M. Lal, Synlett, 2017, 28, 1341-1345.
Quoted from: https://www.organic-chemistry.org/chemicals/reductions/iron.shtm
Aladdinsci: https://www.aladdinsci.com